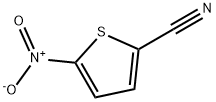
5-NITROTHIOPHENE-2-CARBONITRILE synthesis
- Product Name:5-NITROTHIOPHENE-2-CARBONITRILE
- CAS Number:16689-02-4
- Molecular formula:C5H2N2O2S
- Molecular Weight:154.15
![(NZ)-N-[(5-nitrothiophen-2-yl)methylidene]hydroxylamine](/CAS/20210305/GIF/6030-18-8.gif)
6030-18-8
0 suppliers
inquiry

16689-02-4
136 suppliers
$8.00/1g
Yield:16689-02-4 83%
Reaction Conditions:
with (E)-ethyl 2-cyano-2-(2-nitrophenylsulfonyloxyimino)acetate;1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane at 20;Inert atmosphere;
Steps:
Representative procedure for nitrile synthesis
General procedure: In an oven-dried two-necked 50 mLround-bottomed flask, equipped with a stirring bar, a solution of the oxime(1.0 mmol) and 2-NO2-C6H4-SO3XY(1.5 mmol) dissolved in anhydrous CH2Cl2 (5.0 mL) wasplaced under the atmosphere of nitrogen. The reaction mixture was stirred atroom temperature for 5 min, then DBU (2.5 mmol) was added drop wise over 2 min.The reaction mixture became a clear homogeneous solution after addition of DBU.The reaction was monitored by TLC. The reaction mixture was diluted with EtOAcand washed with water (2×5 mL) followed by brine (2×5 mL) upon completeconsumption of the starting material. Product was purified by columnchromatography.Furthermore, the by-product Oxymacould be readily recovered by acidifying the aqueous layer, and then extractingwith ethyl acetate. The Oxyma thus recovered can then be reused to regeneratethe sulfonate ester of Oxyma, which can be further used for a separate batch ofreaction.
References:
Dev, Dharm;Palakurthy, Nani Babu;Kumar, Nitesh;Mandal, Bhubaneswar [Tetrahedron Letters,2013,vol. 54,# 33,p. 4397 - 4400] Location in patent:supporting information
![2-Thiophenecarboxaldehyde, 5-nitro-, oxime, [C(E)]-](/CAS/20211123/GIF/104416-19-5.gif)
104416-19-5
0 suppliers
inquiry

16689-02-4
136 suppliers
$8.00/1g
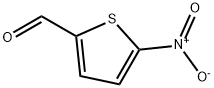
4521-33-9
163 suppliers
$10.00/5g

16689-02-4
136 suppliers
$8.00/1g
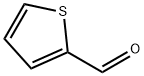
98-03-3
530 suppliers
$5.00/25g

16689-02-4
136 suppliers
$8.00/1g
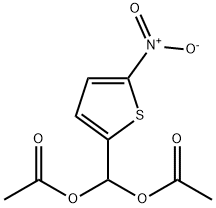
14289-24-8
7 suppliers
inquiry

16689-02-4
136 suppliers
$8.00/1g