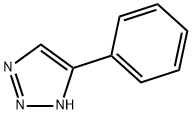
5-PHENYL-1H-1,2,3-TRIAZOLE synthesis
- Product Name:5-PHENYL-1H-1,2,3-TRIAZOLE
- CAS Number:1680-44-0
- Molecular formula:C8H7N3
- Molecular Weight:145.16

100-52-7
954 suppliers
$17.30/2g

1680-44-0
82 suppliers
$57.00/5mg
Yield: 73%
Reaction Conditions:
Stage #1:benzaldehyde with sodium azide;sodium hydrogen sulphite;sodium sulfite in dimethyl sulfoxide at 110;Inert atmosphere;
Stage #2: with nitromethane in dimethyl sulfoxide for 5 h;Inert atmosphere;Reagent/catalyst;Temperature;Solvent;
Steps:
To a 10 mL round-bottom bottle with a condenser was addedbenzaldehyde (2.0 mmol), NaN3 (8.0 mmol), NaHSO3 (2 mmol),Na2SO3 (2 mmol), and DMSO (4 mL). The top of the condenserwas connected to an aq NaOH (1 M) solution via a gas bubbler totrap off any volatile HN3. This reaction mixture was heated at110 °C under argon atmosphere. Then, MeNO2 (4.0 mmol) inDMSO (2 mL) was added by a syringe pump over 2 h; afteradded completely, the mixture was heated for another 3 h. Themixture was cooled to r.t. and poured in to 40 mL H2O, and thenextracted with EtOAc (40 mL). The combined organic phase waswashed by H2O (3 × 40 mL) and sat. brine and then dried overanhydrous Na2SO4. The solvent was removed under reducedpressure, and the crude reaction mixture was purified by flashsilica gel chromatography with PE-EtOAc as an eluent to givethe desired product 3a; white solid; mp 143-145 °C. 1H NMR(400 MHz, DMSO-d6): δ = 15.14 (br, 1 H), 8.33 (br, 1 H), 7.89-7.82 (m, 2 H), 7.47-7.42 (m, 2 H), 7.36 (t, J = 7.2 Hz, 1 H) ppm.13C NMR (100 MHz, DMSO-d6): δ = 128.9, 128.1, 125.6 ppm. IR(KBr): 3158, 2956, 1653, 1457, 1079, 764 cm-1. ESI-HRMS: m/z[M + H]+ calcd for C8H8N3: 146.0718; found: 146.0710.
References:
Wu, Luyong;Wang, Xianghui;Chen, Yuxue;Huang, Qinglan;Lin, Qiang;Wu, Mingshu [Synlett,2016,vol. 27,# 3,art. no. ST-2015-W0410-L,p. 437 - 441]
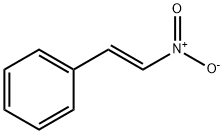
5153-67-3
138 suppliers
$6.00/1g

1680-44-0
82 suppliers
$57.00/5mg
![[(Z)-2-bromoethenyl]benzene](/CAS/GIF/588-72-7.gif)
588-72-7
5 suppliers
inquiry

1680-44-0
82 suppliers
$57.00/5mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1680-44-0
82 suppliers
$57.00/5mg
