5-PHENYL-3,4-DIHYDRO-2H-PYRROLE synthesis
- Product Name:5-PHENYL-3,4-DIHYDRO-2H-PYRROLE
- CAS Number:700-91-4
- Molecular formula:C10H11N
- Molecular Weight:145.2
Yield: 47%
Reaction Conditions:
Stage #1:bromobenzene with magnesium in diethyl ether at 40; for 2 h;
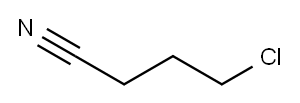
Stage #2:4-Chlorobutyronitrile in diethyl ether at 20 - 40; for 1.5 h;
Steps:
1 General procedure for the synthesis of 2-aryl-1-pyrrolines
General procedure: This procedure was adapted from the literature.28 Unless stated otherwise, the Grignard reagent was prepared by adding dropwise the corresponding aryl bromide to a suspension of excess of magnesium turnings and a few iodine crystals in diethyl ether. The solution was kept for 2h at 40°C. The heating was turned off and a solution of an equimolar amount of 4-chlorobutyronitrile in diethyl ether was added dropwise to the in situ prepared Grignard reagent. The reaction mixture was refluxed for 1.5h and the temperature was then increased up to 110°C to distil off the diethyl ether while xylene was added to keep the total volume constant. The mixture was allowed to cool to room temperature, and a saturated aqueous solution of NH4Cl was added, which caused phases separation. The aqueous phase was repeatedly washed with diethyl ether and the combined organic phases were acidified with a 10% HCl aqueous solution. After extraction, the aqueous acidic solution was neutralised with a NaOH 1M aqueous solution, in an ice bath. Diethyl ether was added and the organic layer extracted and washed with distilled water. After drying over anhydrous MgSO4, the diethyl ether was removed under vacuum and the remaining product purified by trap-to-trap distillation, column chromatography or recrystallization in the appropriate solvent.
4.2.1
2-Phenyl-1-pyrroline (1a)
The phenyl Grignard reagent was prepared from bromobenzene (39.5 mL, 375 mmol) and magnesium turnings (9.24 g, 380 mmol) in diethyl ether (250 mL).
Addition of a solution of 4-chlorobutyronitrile (23.2 mL, 375 mmol) in diethyl ether (250 mL) and subsequent reaction afforded a dark-brown oil, which was purified by trap-to-trap distillation (80 °C and 10-1 mbar).
The resulting light yellow oil was stored at 4 °C, yielding 25.8 g (47%) of 1a as colourless crystals. 1H NMR (300 MHz, CDCl3): δ=7.83-7.80 (m, 2H, Ph-Hortho), 7.38-7.35 (m, Ph-Hmeta and Ph-Hpara), 4.09-4.03 (m, 2H, H5 pyrr), 2.97-2.90 (m, 2H, H3 pyrr), 2.08-1.96 (m, 2H, H4 pyrr) ppm.
References:
Figueira, Cláudia A.;Lopes, Patrícia S.;Gomes, Pedro T. [Tetrahedron,2015,vol. 71,# 25,p. 4362 - 4371]
116437-41-3
63 suppliers
$13.00/100mg

700-91-4
37 suppliers
inquiry
69573-41-7
0 suppliers
inquiry

700-91-4
37 suppliers
inquiry

125330-80-5
10 suppliers
inquiry

700-91-4
37 suppliers
inquiry