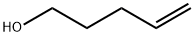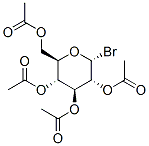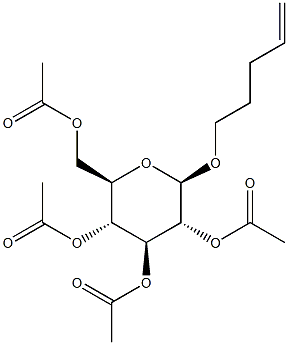
4-Penten-1-yl 2,3,4,6-tetra-O-acetyl-b-D-glucopyranoside synthesis
- Product Name:4-Penten-1-yl 2,3,4,6-tetra-O-acetyl-b-D-glucopyranoside
- CAS Number:50256-33-2
- Molecular formula:C19H28O10
- Molecular Weight:416.4196
Yield:50256-33-2 87%
Reaction Conditions:
with metal carbonates in neat (no solvent); for 3 h;Milling;
Steps:
General procedure for the metal carbonate-assistedglycoside synthesis by planetary ball-milling as typical forpropargyl glycoside
General procedure: The glycosyl bromide (1 mmol), propargyl alcohol (or another acceptor alcohol desired, 2 mmol) and CdCO3/ZnCO3 (1.5 mmol)were allowed to mix in a stainless steel (SS) jar (capacity, 50 mL)containing 10 SS balls (10 mm o.d.) for 2 h (or until the reactionis complete by TLC, depending upon the glycosyl bromide employedas substrate as in Table 2) in a planetary ball mill (RetschPM-100, Retsch GmbH, Germany) at 600 rpm. The reaction mixture was dissolved in CH2Cl2 (or EtOAc, preferred as a greener alternative,is equally effective) and filtered through a Celite-bed and was concentrated under reduced pressure to afford the respective crude propargyl glycoside, which was purified by column chromatography to yield analytically pure product. The spectral data were in accordance with the expected structure and in agreement withthe literature values.
References:
Tyagi, Mohit;Khurana, Darpan;Ravindranathan Kartha [Carbohydrate Research,2013,vol. 379,p. 55 - 59]
604-69-3
394 suppliers
$10.00/25g

50256-33-2
2 suppliers
inquiry

2280-44-6
12 suppliers
inquiry

50256-33-2
2 suppliers
inquiry