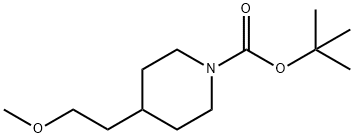
tert-butyl 4-(2-Methoxyethyl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(2-Methoxyethyl)piperidine-1-carboxylate
- CAS Number:509147-79-9
- Molecular formula:C13H25NO3
- Molecular Weight:243.34
Yield:509147-79-9 94%
Reaction Conditions:
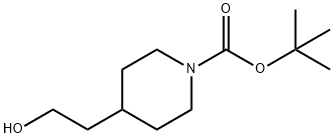
Stage #1: 4-(2-hydroxyethyl)piperidine-1-carboxylic acid tert-butyl esterwith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide; for 18 h;
Steps:
Sodium hydride (202 mg, 8.0 mmol, 95%) was added in portions to a solution of tert-butyl-4- (2-hydroxyethyl) piperidine-1-carboxylate (917 mg, 4.0 mmol) in DMF (5 mL). The mixture was stirred at room temperature for 0.5 h and methyl iodide (1.14 g, 8.0 mmol) in DMF (3 mL) was added slowly. The reaction was quenched after 18 h by addition of water (10 mL), and the resulting aqueous phase was extracted with EtOAc (3x20 mL). The combined organic phases were washed with brine (2x5 mL), dried over MgSO4, and concentrated in vacuo to yield 1.177 g of crude material as a light yellow oil. Purification by flash chromatography (silica, 10-20% EtOAc in n-hexane) yielded 912 mg (94%) of pure tert-butyl 4-(2- methoxyethyl)piperidine-1-carboxylate as a colorless oil.1H NMR (600 MHz, CD3OD) δ ppm 4.04 (d, J=13.4 Hz, 2 H) 3.45 (t, J=6.4 Hz, 2 H) 3.31 (s, 3 H) 2.73 (br. s., 2 H) 1.69 (d, J=12.5 Hz, 2 H) 1.54 - 1.64 (m, 1 H) 1.51 (q, J=6.6 Hz, 2 H) 1.45 (s, 9 H) 1.07 (qd, J=12.2, 4.4 Hz, 2 H). MS (ESI+) m/z 188 [M+H-t-Bu] + .
References:
WO2018/11138,2018,A1 Location in patent:Page/Page column 115-116

74-88-4
340 suppliers
$15.00/10g

509147-79-9
11 suppliers
inquiry