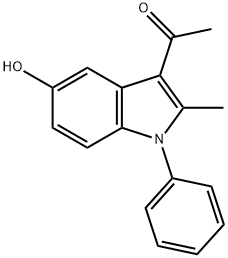
1-(5-HYDROXY-2-METHYL-1-PHENYL-1H-INDOL-3-YL)-ETHANONE synthesis
- Product Name:1-(5-HYDROXY-2-METHYL-1-PHENYL-1H-INDOL-3-YL)-ETHANONE
- CAS Number:5102-18-1
- Molecular formula:C17H15NO2
- Molecular Weight:265.31

62-53-3
636 suppliers
$10.00/1g
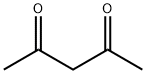
123-54-6
566 suppliers
$10.00/25ml
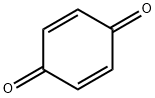
106-51-4
478 suppliers
$10.00/5g

5102-18-1
18 suppliers
$16.00/250mg
Yield:5102-18-1 94%
Reaction Conditions:
Stage #1: aniline;acetylacetonewith montmorillonite KSF clay in 1,2-dichloro-ethane; for 0.25 h;Reflux;Green chemistry;Nenitzescu Synthesis;
Stage #2: p-benzoquinone in 1,2-dichloro-ethane; for 1 h;Reflux;Green chemistry;Nenitzescu Synthesis;
Steps:
Representative Procedure for the Synthesis of 5-Hydroxyindole Derivatives.
General procedure: A mixture of aniline (1 mmol),dimedone (1 mmol) and montmorillonite KSF clay (0.5 g) in1,2-dichloroethane (5 mL) was kept under reflux for 15 min. To this mixture,1,4-benzoquinone (1 mmol) was added and the resulting mixture was allowed to stir under reflux for the appropriate time (Table 1). After completion of the reaction as indicated by TLC, the mixture was filtered and washed with dichloromethane (10 mL). The combined organic layers were concentrated in vacuo and the resulting residue was purified by column chromatography on silica gel (Merck, 60-120 mesh, ethyl acetate-hexane,1:9) to afford the pure 5-hydroxyindole derivative.
References:
Reddy, B.V.Subba;Reddy, P. Sivaramakrishna;Reddy, Y. Jayasudhan;Bhaskar;Reddy, B. Chandra Obula [Bulletin of the Korean Chemical Society,2013,vol. 34,# 10,p. 2968 - 2972]
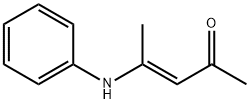
147054-81-7
14 suppliers
inquiry

106-51-4
478 suppliers
$10.00/5g

5102-18-1
18 suppliers
$16.00/250mg

147054-81-7
14 suppliers
inquiry

106-51-4
478 suppliers
$10.00/5g

28241-99-8
38 suppliers
$193.00/1g

5102-18-1
18 suppliers
$16.00/250mg