
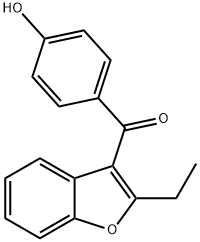
(3,5-Dibromo-4-methoxyphenyl)(2-ethyl-3-benzofuranyl)-methanone synthesis
- Product Name:(3,5-Dibromo-4-methoxyphenyl)(2-ethyl-3-benzofuranyl)-methanone
- CAS Number:51073-13-3
- Molecular formula:C18H14Br2O3
- Molecular Weight:438.11
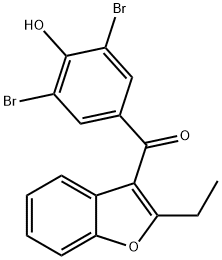
3562-84-3
321 suppliers
$31.00/500mg

74-88-4
351 suppliers
$15.00/10g

51073-13-3
18 suppliers
inquiry
Yield:51073-13-3 100%
Reaction Conditions:
with potassium carbonate in acetone; for 16 h;Heating / reflux;
Steps:
8
Compound 2. A heterogeneous mixture of 1 (26.8 g, 60.0 mmol), K2CO3 (33.5 g, 240 mmol), and methyl iodide (12.9 g, 90.0 mmol) in acetone (200 mL) was heated to reflux for 16 hours. Ethyl acetate (300 mL) was added, and the mixture was extracted sequentially with water (200 mL), 2.5 M NaOH (2*100 mL), and brine (100 mL). The organic portion was dried over Na2SO4 and concentrated to give 26.3 g (100% yield) of the title compound as a white solid. <1>H NMR (CDCl3): [delta]1.36 (t, J=7.5 Hz, 3 H), 2.90 (q, J=7.5 Hz, 2 H), 3.98 (s, 3 H), 7.24 (app t, J=7.4 Hz, 1 H), 7.32 (app t, J=7.2 Hz, 1 H), 7.42 (d, J=7.2 Hz, 1 H), 7.50 (d, J=7.9 Hz, 1 H), 7.99 (s, 2 H). LRMS: ion 438 (M+).
References:
US2003/195247,2003,A1 Location in patent:Page/Page column 13

3562-84-3
321 suppliers
$31.00/500mg
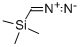
18107-18-1
211 suppliers
$33.00/5g

51073-13-3
18 suppliers
inquiry
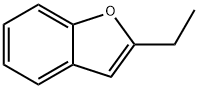
3131-63-3
181 suppliers
$10.00/5g

51073-13-3
18 suppliers
inquiry
1477-19-6
142 suppliers
$15.00/100mg

51073-13-3
18 suppliers
inquiry
3343-80-4
16 suppliers
inquiry

51073-13-3
18 suppliers
inquiry