ethyl 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate synthesis
- Product Name:ethyl 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate
- CAS Number:52505-51-8
- Molecular formula:C11H12N2O2S
- Molecular Weight:236.29
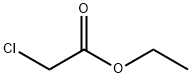
28900-10-9
236 suppliers
$9.00/1g

623-51-8
228 suppliers
$7.00/25g
![ethyl 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate](/CAS/GIF/52505-51-8.gif)
52505-51-8
21 suppliers
$46.00/1g
Yield:52505-51-8 97%
Reaction Conditions:
with sodium ethanolate in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
52
Reference Example 52 ethyl 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate To a solution of 2-chloro-6-methylnicotinonitrile (44.0 g, 0.289 mol) and ethyl thioglycolate (35.0 mL, 0.318 mol) in DMF (500 mL) was added sodium ethoxide (21.7 g, 0.318 mol), and the mixture was stirred at room temperature for 30 min. Sodium ethoxide (5.00 g, 73.5 mmol) was added again, and the mixture was stirred at room temperature for 30 min. Water was added to the reaction mixture, and the precipitated solid was collected by filtration, washed with water, and dried to give the title compound (66.4 g, yield 97%) as a yellow solid. 1H NMR (CDCl3) δ1.39 (3H, t, J = 7.2 Hz), 2.68 (3H, s), 4.36 (2H, q, J = 7.2 Hz), 5.89 (2H, br), 7.16 (1H, d, J = 8.3 Hz), 7.81 (1H, d, J = 8.3 Hz).
References:
EP2009011,2008,A1 Location in patent:Page/Page column 41
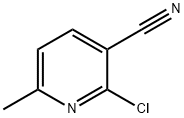
40381-90-6
191 suppliers
$6.00/250mg
![ethyl 3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate](/CAS/GIF/52505-51-8.gif)
52505-51-8
21 suppliers
$46.00/1g