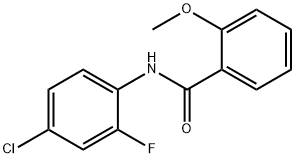
N-(2-Fluoro-4-chlorophenyl)-2-MethoxybenzaMide, 97% synthesis
- Product Name:N-(2-Fluoro-4-chlorophenyl)-2-MethoxybenzaMide, 97%
- CAS Number:546096-42-8
- Molecular formula:C14H11ClFNO2
- Molecular Weight:279.69

1308631-40-4
28 suppliers
$28.00/1mg

74-88-4
340 suppliers
$15.00/10g

546096-42-8
1 suppliers
inquiry
Yield:546096-42-8 81%
Reaction Conditions:
with potassium carbonate in acetone; for 8 h;Reflux;
Steps:
2 N-(4-chloro-2-fluorophenyl)-2-methoxybenzamide (compound 1b)
To a solution of compound 1a (0.53 g, 2 mmole) in anhydrous acetone (10 mL) were added potassium carbonate (0.69 g, 5 mmole) and iodomethane (0.28 mL, 4.4 mmole) and refluxed 8 hr. After cooled to room temperature, the reaction mixture was filtered through Celite and concentrated. The residue was extracted with ethyl acetate and dried over anhydrous magnesium sulfate, then concentrated and recrystallized with hot methanol. The pure compound was obtained as white powder (yield 81%). Mp 111-112° C. 1H NMR (300 MHz, CDCl3): ppm 4.07 (s, 3H), 7.05 (d, J=8.4 Hz, 1H), 7.02-7.18 (m, 3H), 7.49-7.55 (m, 1H), 8.29 (dd, J=7.8, 1.8 Hz, 1H), 8.55-8.61 (m, 1H), 10.35 (br, 1H). HRMS (EI) m/z calcd for C14H11ClFNO2+ [M]+: 279.0462. Found: 279.0458
References:
US2013/281412,2013,A1 Location in patent:Paragraph 0052; 0053
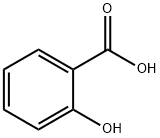
69-72-7
1227 suppliers
$5.00/10g

546096-42-8
1 suppliers
inquiry
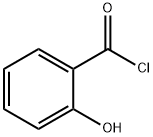
1441-87-8
30 suppliers
$335.77/1gm:

546096-42-8
1 suppliers
inquiry