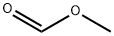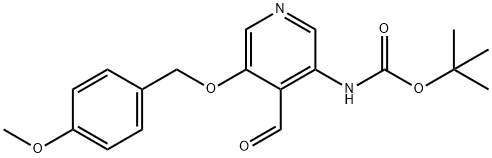
[4-Formyl-5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic acid tert-butyl ester synthesis
- Product Name:[4-Formyl-5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic acid tert-butyl ester
- CAS Number:552331-77-8
- Molecular formula:C19H22N2O5
- Molecular Weight:358.39
Yield:552331-77-8 63%
Reaction Conditions:
Stage #1: [5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic acid tert-butyl esterwith n-butyllithium in tetrahydrofuran;hexane at -78 - -10; for 0.75 h;
Stage #2: Methyl formate in tetrahydrofuran;hexane at -78; for 0.166667 h;
Steps:
234.D [4-Formyl-5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic Acid Tert-Butyl Ester
Example 234D [4-Formyl-5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic Acid Tert-Butyl Ester To Example 234C (2.50 g, 7.54 mmol) in THF (125 mL) at -78° C. was added dropwise via syringe n-BuLi (6.64 mL of a 2.5 M solution in hexanes, 16.6 mmol). The resulting dark orange reaction mixture was slowly warmed to -10° C. over 45 min and then recooled to -78° C. Methyl formate (1.40 mL, 22.6 mmol) was added dropwise via syringe and the reaction was stirred an additional 10 min at -78° C. Saturated aqueous NH4Cl was added and the quenched reaction mixture warmed to room temperature. H2O and Et2O were added and the layers separated. The organics were washed with brine and dried over MgSO4. Flash chromatography (20-40-60-80% EtOAc/hexanes gave 0.3 g (12%) of recovered starting material, and 1.7 g (63%) of as a yellow solid. (1H NMR (400 MHz, DMSO-D6) δ ppm 1.47 (s, 9H) 3.75 (s, 3H) 5.28 (s, 2H) 6.95 (d, J=8.59 Hz, 2H) 7.43 (d, J=8.59 Hz, 2H) 8.38 (s, 1H) 9.07 (s, 1H) 10.17 (s, 1H) 10.35 (s, 1H), 13C NMR (100 MHz, DMSO-D6) δ ppm 27.7, 55.0, 70.8, 81.1, 113.9, 114.6, 127.7, 129.6, 130.0, 133.3, 134.6, 151.7, 154.7, 159.2, 192.8, Anal Calcd for C19H22N2O5: C, 63.67; H, 6.19; N, 7.82. Found: C: 63.59, H: 6.21, N: 7.64.
References:
US2003/199511,2003,A1 Location in patent:Page/Page column 70
![3-Pyridinamine, 5-[(4-methoxyphenyl)methoxy]-](/CAS/20210305/GIF/552331-74-5.gif)
552331-74-5
0 suppliers
inquiry
![[4-Formyl-5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic acid tert-butyl ester](/CAS/GIF/552331-77-8.gif)
552331-77-8
8 suppliers
inquiry

74115-13-2
396 suppliers
$10.00/1g
![[4-Formyl-5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic acid tert-butyl ester](/CAS/GIF/552331-77-8.gif)
552331-77-8
8 suppliers
inquiry
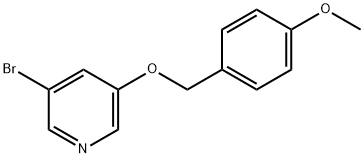
552331-73-4
26 suppliers
$55.00/250mg
![[4-Formyl-5-(4-methoxy-benzyloxy)-pyridin-3-yl]-carbamic acid tert-butyl ester](/CAS/GIF/552331-77-8.gif)
552331-77-8
8 suppliers
inquiry
![Carbamic acid, [5-[(4-methoxyphenyl)methoxy]-3-pyridinyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20210305/GIF/552331-75-6.gif)