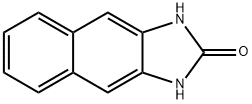
2H-Naphth[2,3-d]imidazol-2-one,1,3-dihydro-(8CI,9CI) synthesis
- Product Name:2H-Naphth[2,3-d]imidazol-2-one,1,3-dihydro-(8CI,9CI)
- CAS Number:5649-76-3
- Molecular formula:C11H8N2O
- Molecular Weight:184.19
Yield: 84%
Reaction Conditions:
for 0.25 h;Microwave irradiation;
Steps:
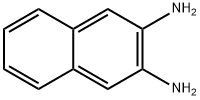
lH-naphtho[2,3-d] imidazol-2(3H)-one (7).
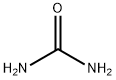
lH-naphtho[2,3-d] imidazol-2(3H)-one (7). According to a previously described method, (Kidwai et al., 2005) 2,3-diaminonapthalene (430 mg, 2.77 mmol) and urea (218 mg, 3.63 mmol) were added to a 100 mL Erlenmeyer flask. The flask was heated for 15 min in a 700W kitchen microwave, with stopping every 5 minutes to break up and stir the chunky bronze-colored reaction mixture. The product was suspended in 5% aqueous HCl, and the precipitate was filtered off and washed twice more with 5% HCl and twice with water. The gold-colored solid was then dried under vacuum to yield product (84% yield). NMR (DMSO-de): 10.797 (s, 1.9 H), 7.80 (m, 2H), 7.28 (m, 4H). 13C NMR (DMSO-de): 156.14, 131.00, 129.16, 123.22, 103.55. ESI-MS: M+H+ calc'd 185.0709, found 185.0711.
References:
ETH ZURICH;STURLA, Shana;TRANTAKIS, Ioannis;DAHLMANN, Heidi WO2015/162130, 2015, A1 Location in patent:Page/Page column 29-30
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2H-Naphth[2,3-d]imidazol-2-one,1,3-dihydro-(8CI,9CI)](/CAS/20180808/GIF/5649-76-3.gif)
5649-76-3
6 suppliers
inquiry