
3-(2-methoxyphenyl)-2-sulfanylidene-thiazolidin-4-one synthesis
- Product Name:3-(2-methoxyphenyl)-2-sulfanylidene-thiazolidin-4-one
- CAS Number:56676-49-4
- Molecular formula:C10H9NO2S2
- Molecular Weight:239.31

75-15-0
253 suppliers
$40.00/100g
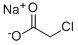
3926-62-3
288 suppliers
$19.73/250g
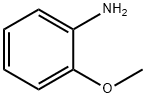
90-04-0
383 suppliers
$5.00/5G

56676-49-4
10 suppliers
$1000.00/5G
Yield:56676-49-4 63%
Reaction Conditions:
Stage #1: carbon disulfide;2-methoxy-phenylaminewith triethylamine in ethanol at 0; for 0.5 h;
Stage #2: sodium monochloroacetic acid in water at 20;
Stage #3: with hydrogenchloride in water; for 0.0833333 h;
Steps:
General procedure: Cyclohexanamine 6a (0.5g, 5mmol), and triethylamine (2.35g, 25mmol) were solved in ethanol (2ml), then carbon disulfide (0.76g, 10mmol)was dropped into mixture with stirring for 0.5h at 0 °C. The precipitate was filtered and washed by ether and solved in solution of sodium chloroacetate (0.64g, 5.5mmol) at RT overnight. 6M HCl (7ml)was added to reaction mixture and heated at 95 °C for 5min. The crude product was filtered and purified by recrystallization with chloroform to give the yellow solid as target compound
References:
Liang, Xiao;Fu, Huansheng;Xiao, Peng;Fang, Hao;Hou, Xuben [Bioorganic Chemistry,2020,vol. 103,art. no. 104124]

2365-48-2
357 suppliers
$14.00/25g
3288-04-8
86 suppliers
$11.00/1g

56676-49-4
10 suppliers
$1000.00/5G

90-04-0
383 suppliers
$5.00/5G

56676-49-4
10 suppliers
$1000.00/5G
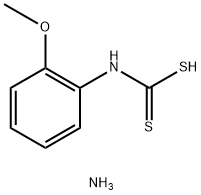
49791-47-1
0 suppliers
inquiry

56676-49-4
10 suppliers
$1000.00/5G

6326-83-6
110 suppliers
$21.00/5g

90-04-0
383 suppliers
$5.00/5G

56676-49-4
10 suppliers
$1000.00/5G