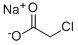
Sodium chloroacetate synthesis
- Product Name:Sodium chloroacetate
- CAS Number:3926-62-3
- Molecular formula:C2H2ClNaO2
- Molecular Weight:116.48
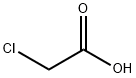
79-11-8
0 suppliers
$24.00/25g
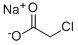
3926-62-3
291 suppliers
$16.69/250g
Yield:-
Reaction Conditions:
with sodium carbonate in water at 45;
Steps:
A3.1
A3. i-Acetyl-θ-chloro-IH-indol-S-yl acetateStep 1 : 2-[(Carboxymethyl)amino]-4-chlorobenzoic acid. Chloroacetic acid (15 g) is dissolved in water (15 ml) and sodium carbonate (16 g) is added in portions (carbon dioxide evolves) and the mixture is stirred at 450C for 30 min. In parallel, 2-amino-4-chlorobenzoic acid is dissolved in water (15 ml), 1 M aqueous sodium hydroxide solution (15 ml) is added, followed by the sodium chloroacetate solution prepared above. The resulting mixture is stirred at 6O0C for 2 days. After cooling, 1 M aqueous sodium hydroxide solution is added, after that, it is acidified by addition of concentrated hydrochloric acid. The yellow precipitate, that is formed, is filtered off and washed with water (100 ml). It is redissolved in tetrahydrofuran / ethyl acetate (1 :1 (v/v), 150 ml), dried (Na2SO4) and concentrated in vacuo. The residue, 20.0 g of crude 2-[(carboxymethyl)amino]-4-chlorobenzoic acid as a yellow solid, is used without further purification in the next step.
References:
NYCOMED GMBH;BARTELS, Björn;WEINBRENNER, Steffen;MARX, Degenhard;DIEFENBACH, Jörg;DUNKERN, Torsten;MENGE, Wiro M.P.B.;CHRISTIAANS, Johannes A. M. WO2010/15589, 2010, A1 Location in patent:Page/Page column 107

79-11-8
0 suppliers
$24.00/25g
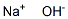
1310-73-2
1399 suppliers
$14.00/25g
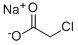
3926-62-3
291 suppliers
$16.69/250g