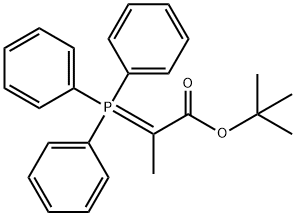
Propanoic acid, 2-(triphenylphosphoranylidene)-, 1,1-dimethylethyl ester synthesis
- Product Name:Propanoic acid, 2-(triphenylphosphoranylidene)-, 1,1-dimethylethyl ester
- CAS Number:56904-86-0
- Molecular formula:C25H27O2P
- Molecular Weight:390.45
![Phosphonium, [2-(1,1-dimethylethoxy)-1-methyl-2-oxoethyl]triphenyl-, bromide (1:1)](/CAS/20211123/GIF/849240-08-0.gif)
849240-08-0
0 suppliers
inquiry

56904-86-0
0 suppliers
inquiry
Yield:56904-86-0 16.8 g
Reaction Conditions:
with sodium hydroxide in dichloromethane;water;
Steps:
50 Example 50. Synthesis of tert-butyl 2-(triphenylphosphoranylidene)propanoate (184)
A mixture of tert-butyl-2-bromopropanoate 183 (15.5 g, 74.1 mmol, 1.0 eq.) and triphenyl phosphine (19.4 g, 74.1 mmol, 1.0 eq.) in dry acetonitrile (45 mL) was stirred at room temperature for 18 h. Acetonitrile was removed under reduced pressure and toluene was added to crash out a white precipitate. Toluene was then decanted off and the white solid was dissolved in dichloromethane (100 mL) and transferred to a separatory funnel.10% NaOH (100 mL) was added to the funnel, and the organic layer immediately turned yellow after shaking. The organic layer was separated and the aqueous layer was extracted with dichloromethane (30 mL) once. The dichloromethane layers were combined and washed with brine (50 mL) once, then dried over Na2SO4, filtered and concentrated, giving the ylide as a yellow solid (16.8 g, 58%).
References:
WO2016/59622,2016,A2 Location in patent:Page/Page column 110
603-35-0
698 suppliers
$5.00/5G

56904-86-0
0 suppliers
inquiry
56905-19-2
0 suppliers
inquiry

56904-86-0
0 suppliers
inquiry
![Phosphonium, [2-(1,1-dimethylethoxy)-2-oxoethyl]triphenyl-, inner salt](/CAS/20211123/GIF/86302-43-4.gif)
