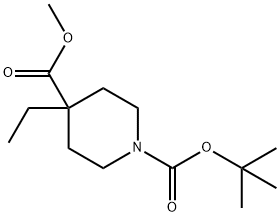
1,4-Piperidinedicarboxylic acid, 4-ethyl-, 1-(1,1-dimethylethyl) 4-methyl ester synthesis
- Product Name:1,4-Piperidinedicarboxylic acid, 4-ethyl-, 1-(1,1-dimethylethyl) 4-methyl ester
- CAS Number:578021-55-3
- Molecular formula:C14H25NO4
- Molecular Weight:271.35
124443-68-1
263 suppliers
$6.00/5g

75-03-6
354 suppliers
$11.00/5g

578021-55-3
59 suppliers
$45.00/100mg
Yield:578021-55-3 95.58%
Reaction Conditions:
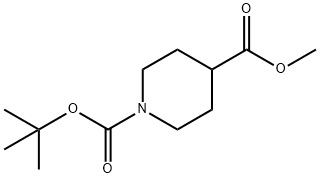
Stage #1: 1-tert-Butyl 4-methyl piperidine-1,4-dicarboxylatewith lithium diisopropyl amide in tetrahydrofuran at -78; for 0.75 h;Inert atmosphere;
Stage #2: ethyl iodide in tetrahydrofuran at -78 - 20;Inert atmosphere;
Steps:
6 Synthesis of 1-tert-butyl 4-methyl 4-ethylpiperidine-l,4-dicarboxylate:
Into a (0519) 250-mL 3-necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen, was placed 1-tert-butyl 4-methyl piperidine-l,4-dicarboxylate (10.00 g, 41.101 mmol, 1.00 equiv), THF (100.00 mL). This was followed by the addition of LDA (61.65 mL, (0520) 61.650 mmol, 1.50 equiv) dropwise with stirring at -78 degrees C. The resulting solution was stirred for 45 min at -78 degrees C. To this was added ethyl iodide (10.23 g, 65.591 mmol, (0521) 1.60 equiv) dropwise with stirring at -78 degrees C. The resulting solution was stirred overnight at room temperature. The reaction was then quenched by the addition of 500 mL of (0522) NH4CI. The resulting solution was extracted with 2x300 mL of ethyl acetate and the organic layers combined. The resulting mixture was washed with 1 x500 ml of brine. The mixture was dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1:10). This resulted in (0523) 10.66 g (95.58%) of 1-tert-butyl 4-methyl 4-ethylpiperidine-l,4-dicarboxylate as yellow oil. (0524) H-NMR (300 MHz, Chloroform- , ppm ) d 3.88 (dt, J= 13.9, 4.0 Hz, 2H), 3.72 (s, 3H), 2.88 (0525) (ddd, J= 14.1, 11.7, 2.8 Hz, 2H), 2.18 - 2.03 (m, 2H), 1.57 (q, J= 7.5 Hz, 2H), 1.46 (s, 9H), 1.34 (ddd, J= 13.5, 11.6, 4.4 Hz, 2H), 0.83 (t, J= 7.5 Hz, 3H).
References:
WO2021/158948,2021,A1 Location in patent:Page/Page column 54-55

74-96-4
427 suppliers
$9.00/5g

124443-68-1
263 suppliers
$6.00/5g

578021-55-3
59 suppliers
$45.00/100mg