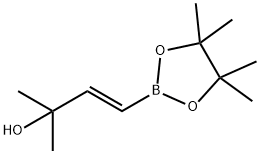
(E)-2-Methyl-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)but-3-en-2-ol synthesis
- Product Name:(E)-2-Methyl-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)but-3-en-2-ol
- CAS Number:581802-26-8
- Molecular formula:C11H21BO3
- Molecular Weight:212.09
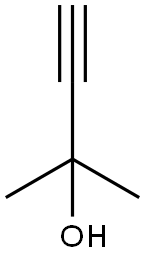
115-19-5
263 suppliers
$20.00/25mL

73183-34-3
572 suppliers
$6.00/5g

581802-26-8
41 suppliers
$45.00/10mg
Yield:581802-26-8 97%
Reaction Conditions:
with sodium methylate;Cu(2+)*4Cu(1+)*4I(1-)*2C5H3N2O2(1-)*C5H5N*C2H7N*3C3H7NO in ethanol at 25; for 1 h;Inert atmosphere;
Steps:
2.3. Typical procedure for the hydroborylation of alkynes
General procedure: The typical experimental procedure for the hydroborylation ofalkynes is as follows: a mixture of alkyne (2 mmol), compound 1(0.006 mmol), bis(pinacolato)diboron (2.5 mmol), NaOMe(0.2 mmol) and 5 mL EtOH were successively added into a reaction flask equipped with a Ar (99.99%) balloon. Then the flask was vacuumedand charged in Ar three times to exclude air in the reactionsystem. Subsequently, the obtained reaction mixture was magneticallystirred at 25 °C for 1 h. After the reaction was completed, thecatalysts were separated by filtration and the yield was determinedby GC analysis. The pure products were obtained by columnchromatography on silica gel (petroleum ether/ethyl acetate as aneluent).
References:
Gao, Ning;Hu, Tianding;Kang, Xiaomin;Lan, Xingwang;Wang, Zhenguang;Wu, Zhi-Lei;Zhao, Bin [Journal of Catalysis,2021,vol. 404,p. 250 - 257]

115-19-5
263 suppliers
$20.00/25mL
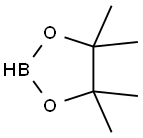
25015-63-8
221 suppliers
$22.39/5G

581802-26-8
41 suppliers
$45.00/10mg
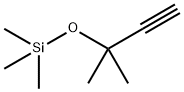
![1,3,2-Dioxaborolane, 4,4,5,5-tetramethyl-2-[(1E)-3-methyl-3-[(trimethylsilyl)oxy]-1-buten-1-yl]-](/CAS/20200611/GIF/1138162-60-3.gif)
1138162-60-3
1 suppliers
inquiry

581802-26-8
41 suppliers
$45.00/10mg
17869-77-1
44 suppliers
$64.50/5ml

25015-63-8
221 suppliers
$22.39/5G

581802-26-8
41 suppliers
$45.00/10mg
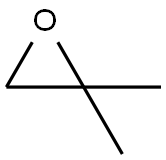
558-30-5
225 suppliers
$20.00/5G
![Bis[(pinacolato)boryl]Methane](/CAS/GIF/78782-17-9.gif)
78782-17-9
95 suppliers
$22.00/1g

581802-26-8
41 suppliers
$45.00/10mg