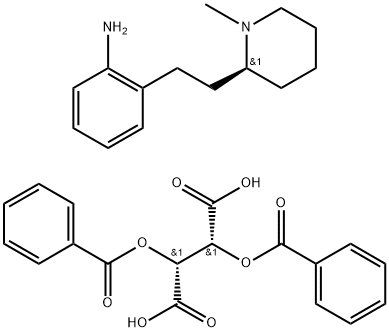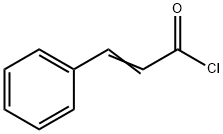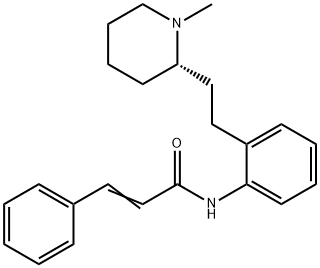
(E)-N-[2-[2-[(2S)-1-methyl-2-piperidyl]ethyl]phenyl]-3-phenyl-prop-2-e namide synthesis
- Product Name:(E)-N-[2-[2-[(2S)-1-methyl-2-piperidyl]ethyl]phenyl]-3-phenyl-prop-2-e namide
- CAS Number:58754-46-4
- Molecular formula:C23H28N2O
- Molecular Weight:348.48
Yield:-
Reaction Conditions:
Stage #1: (S)-[2-(o-aminophenethyl)-1-methylpiperidine dibenzoyl-L-tartrate]with sodium hydrogencarbonate in water;ethyl acetate;
Stage #2: cinnamoyl chloridewith potassium carbonate in ethyl acetate; for 14 h;Reflux;
Steps:
1.D
A solution of S-APEMP.DBLT (287 g, 0.5 mole) in ethyl acetate (3.2 L.) (or other low boiling water immiscible solvent) was extracted with 7.5% aqueous sodium bicarbonate (3.2 L.) to liberate the S-APEMP. After a water wash and drying over a suitable drying agent the solvent was removed in vacuo. The oily residue, S-APEMP, was dissolved in ethyl acetate (1.0 L.) and anhydrous potassium carbonate (412 g, 3.0 moles) (or other suitable acid acceptor such as triethyl amine, pyridine etc.) was added. Cinnamoyl chloride (143 g., 0.7 mole) in 700 ml. of ethyl acetate was added slowly. After the initial reaction, the mixture was refluxed for 14 hrs. After cooling to room temperature the mixture was extracted with water (1.7 L.) and dried over a suitable drying agent. After removing the drying agent the solvent was removed in vacuo and the residue was dissolved in hot ethyl acetate (280 ml.) and allowed to slowly cool to room temperature; filtration yielded S-MPEC, (136 g., 79% yield). Analysis: Calcd. For C, H, N: C, 79.27; H, 8.10; N, 8.04. Found: C, 79.27; H, 8.06; N, 8.07. HPLC(chiral)purity: 99.5%, [∞]D25° = -46° (c=0.01,EtOH); Melting point: 128°C.
References:
WO2012/26914,2012,A1 Location in patent:Page/Page column 13
552-89-6
651 suppliers
$5.00/1g
![(E)-N-[2-[2-[(2S)-1-methyl-2-piperidyl]ethyl]phenyl]-3-phenyl-prop-2-e namide](/CAS/GIF/58754-46-4.gif)
58754-46-4
11 suppliers
inquiry
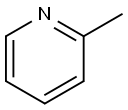
109-06-8
417 suppliers
$16.00/25g
![(E)-N-[2-[2-[(2S)-1-methyl-2-piperidyl]ethyl]phenyl]-3-phenyl-prop-2-e namide](/CAS/GIF/58754-46-4.gif)
58754-46-4
11 suppliers
inquiry
![2-[(Z)-2-(2-nitrophenyl)ethenyl]pyridine](/CAS/20180529/GIF/50385-24-5.gif)
50385-24-5
2 suppliers
inquiry
![(E)-N-[2-[2-[(2S)-1-methyl-2-piperidyl]ethyl]phenyl]-3-phenyl-prop-2-e namide](/CAS/GIF/58754-46-4.gif)
58754-46-4
11 suppliers
inquiry