
5H-1,2,3-Oxathiazole, 4-methyl-, 2,2-dioxide synthesis
- Product Name:5H-1,2,3-Oxathiazole, 4-methyl-, 2,2-dioxide
- CAS Number:1025506-08-4
- Molecular formula:C3H5NO3S
- Molecular Weight:135.14
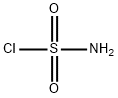
7778-42-9
112 suppliers
$11.00/1g
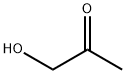
116-09-6
251 suppliers
$11.00/25g

1025506-08-4
3 suppliers
inquiry
Yield:1025506-08-4 72.4 g
Reaction Conditions:
with pyridine in dichloromethane at -5 - 20;Inert atmosphere;
Steps:
32.A.2 4-methyl-5H-1,2,3-oxathiazole 2,2-dioxide
In a separate 5L 4 neck reaction flask was charged hydroxyacetone (72.5 mL, 953 mmol), pyridine (1 16 mL, 1430 mmol), and DCM (2000 mL). This solution was cooled to -5 °C under N2. The sulfamoyl chloride solution was added slowly via Teflon tube over 10 min. After the addition, the reaction was stirred for 15 min then the ice bath was removed and the reaction mixture allowed to warm to room temperature. As the reaction progressed, a gummy material formed. The material was purified via silica gel chromatography (300 g silica gel eluting with DCM). Obtained 4-methyl-5H-1,2,3-oxathiazole 2,2-dioxide (72.4 g, 536 mmol, 56 % yield) as a colorless solid. 1H NMR (400MHz, CHLOROFORM-d) δ 5.09 (s, 2H), 2.44 (s, 3H); LCMS (ESI) m/e 136.0 [(M+H)+, calcd for C3H6NO3S 136.0].
References:
WO2015/153720,2015,A1 Location in patent:Page/Page column 66

116-09-6
251 suppliers
$11.00/25g

1025506-08-4
3 suppliers
inquiry