
6-(1-PYRAZOLYL)-2-QUINOLONE synthesis
- Product Name:6-(1-PYRAZOLYL)-2-QUINOLONE
- CAS Number:102791-46-8
- Molecular formula:C12H9N3O
- Molecular Weight:211.22
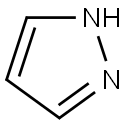
288-13-1
415 suppliers
$10.00/1g
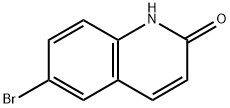
1810-66-8
177 suppliers
$10.00/250mg

102791-46-8
8 suppliers
inquiry
Yield:102791-46-8 50%
Reaction Conditions:
with potassium carbonate;copper(l) iodide in DMF (N,N-dimethyl-formamide) at 160; for 22 h;
Steps:
131.B Example 131; General Procedure (B); 2-(4-Isopropylpiperazin-1-yl)-6-pyrazol-1-ylquinoline hydrochloride
This compound was prepared using the General Procedure (B) from 1-isopropylpiperazine and 2-chloro-6-(1-pyrazolyl)quinoline. The required 2-chloro-6-(1-pyrazolyl)quinoline was prepared by treatment of 6-(1-pyrazolyl)-2-quinolone with POCl3. 6-(1-Pyrazolyl)-2-quinolone was prepared in the following way: [0873] A mixture of 6-bromo-2-quinolone (3.58 g, 16.0 mmol), DMF (15 ml), pyrazole (1.66 g, 24.4 mmol), potassium carbonate (3.33 g, 24.1 mmol), and copper(I) iodide (0.76 g, 3.99 mmol) was stirred at 160° C. for 22 h. Water (300 ml) was added, and after thorough trituration the mixture was filtered, and the solid was washed with water. The solid was stripped with EtOH, resuspended in a mixture of acetonitrile and ethanol (100 ml, 1:1), heated to reflux, and kept at room temperature overnight. Filtration, washing with a bit of acetonitrile, and drying under reduced pressure yielded 1.7 g (50%) of 6-(1-pyrazolyl)-2-quinolone as a metallic-green solid. [0874] 1H NMR (DMSO-d6) δ1.32 (d, J=7 Hz, 6H), 3.10-3.70 (m, 7H), 4.81 (m, 2H), 6.60 (m, 1H), 7.56 (d, J=8 Hz, 1H), 7.81 (s, 1H), 8.09 (m, 1H), 8.22 (d, J=8 Hz, 1H), 8.32 (s, 1H), 8.38 (d, J=8 Hz, 1H), 8.58 (m, 1H), 11.11 (br s, 1H); HPLC-MS: m/z 322 (MH+); Rt=1.67 min.
References:
US2003/236259,2003,A1 Location in patent:Page 56

327058-51-5
32 suppliers
$150.00/250mg

102791-46-8
8 suppliers
inquiry