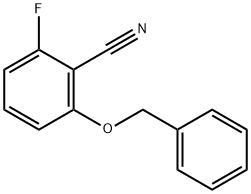
6-benzyloxy-2-fluorobenzonitrile synthesis
- Product Name:6-benzyloxy-2-fluorobenzonitrile
- CAS Number:94088-45-6
- Molecular formula:C14H10FNO
- Molecular Weight:227.23

100-39-0
435 suppliers
$10.00/10g
140675-43-0
186 suppliers
$8.00/250mg

94088-45-6
40 suppliers
inquiry
Yield:94088-45-6 96%
Reaction Conditions:
with potassium carbonate;potassium iodide in acetonitrile at 20; for 12 h;
Steps:
1.1 1. Synthesis of 2-(Benzyloxy)-6-fluorobenzonitrile (Compound 4)
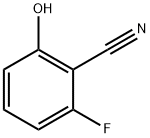
To a mixture of 10.00 g (73.0 mmol, 2.0 eq) K2CO3, 200 mg KI and 5.00 g 2-fluoro-6-hydroxybenzonitrile (36.5 mmol, 1.0 eq) in 100 ml acetonitrile, then 6.55 g (38.3 mmol, 1.05 eq) benzyl bromide was added to the mixture, and the whole was stirred at room temperature for 12 h.
After the reaction was complete, most of the solution was removed under reduced pressure, water was added to the residue and extracted with EA, the organic layers washed with saturated sodium chloride solution, dried over Na2SO4, filtered, and concentrated.
The residue was purified with column chromatography (PE/EA = 15:1) to give compound 4, 8.0 g white solid, yield 96%.
The structure is confirmed correct and data are as follow: MS (ESI) (m/z): 228.0 (M+H)+. 1H NMR (400 MHz, DMSO-d6) δ 7.73 - 7.62 (m, 1H), 7.43 (d, J = 7.0 Hz, 2H), 7.38 (t, J = 7.4 Hz, 2H), 7.32 (d, J = 7.1 Hz, 1H), 7.16 (d, J = 8.7 Hz, 1H), 7.03 (t, J = 8.8 Hz, 1H), 5.27 (s, 2H).
References:
EP3750890,2020,A1 Location in patent:Paragraph 0030-0032
1897-52-5
539 suppliers
$7.00/10g

100-51-6
1410 suppliers
$5.00/100g

94088-45-6
40 suppliers
inquiry