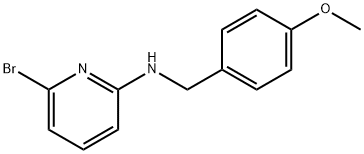
6-BROMO-N-[(4-METHOXYPHENYL)METHYL]-2-PYRIDINAMINE synthesis
- Product Name:6-BROMO-N-[(4-METHOXYPHENYL)METHYL]-2-PYRIDINAMINE
- CAS Number:312263-22-2
- Molecular formula:C13H13BrN2O
- Molecular Weight:293.16
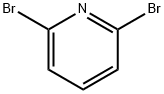
626-05-1
497 suppliers
$9.00/10g
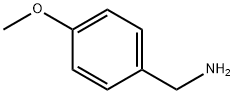
2393-23-9
462 suppliers
$20.00/25g
![6-BROMO-N-[(4-METHOXYPHENYL)METHYL]-2-PYRIDINAMINE](/CAS/GIF/312263-22-2.gif)
312263-22-2
16 suppliers
$258.63/10g
Yield:312263-22-2 91%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in 1,4-dioxane;
Steps:
5.A Step A
Step A (6-Bromo-pyridin-2-yl)-(4-methoxy-benzyl)-amine (6-2) A solution of 2,6-dibromopyridine 6-1 (37.3 g, 157 mmol), 4-methoxybenzylamine (21.6 g, 157 mmol), and diisopropylethylamine (22.4 g, 173 mmol) in 1,4-dioxane (150 mL) was heated at reflux for 24 h. The reaction mixture was cooled, concentrated in vacuo, and the residue was partitioned between EtOAc (100 mL) and saturated aqueous NaHCO3 (125 mL). The organic layer was washed with water (100 mL) followed by brine (100 mL) and dried (Na2SO4). The organic layer was filtered, concentrated in vacuo, and purified by flash chromatography (silica, 10% to 40% EtOAc/hexanes) affording 42 g of amine 6-2 as a white solid in 91% yield. 1H NMR (300 MHz, CDCl3) δ 7.26-7.22 (m, 3H), 6.88-6.86 (m, 2H), 6.75-6.73 (d, 1H), 6.27-6.25 (d, 1H), 4.98-4.96 (br s, 1H), 4.39-4.37 (d, 2H), 3.80 (s, 3H).
References:
US2001/53853,2001,A1