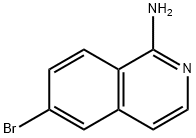
6-BROMOISOQUINOLIN-1-YLAMINE synthesis
- Product Name:6-BROMOISOQUINOLIN-1-YLAMINE
- CAS Number:215453-26-2
- Molecular formula:C9H7BrN2
- Molecular Weight:223.07
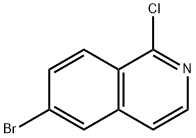
205055-63-6
207 suppliers
$24.00/250mg

215453-26-2
110 suppliers
$50.00/25 mg
Yield: 95%
Reaction Conditions:
with ammonium hydroxide in 1-methyl-pyrrolidin-2-one;water at 150; for 10 h;Inert atmosphere;Solvent;
Steps:
51a; 51b Examples 51a and 51b. Preparation of (S)-2-(i-(methylamino)isoquinolin-6- yl)pyrrolidine- 1-carbonitrile (51 a) and (R)-2-(1-(methylamino)isoquinolin-6-yl)pyrrolidine- 1-carbonitrile (Sib)
To a solution of compound 51.8 (2.5 g, 10.31 mmol, 1 eq) in NMP (30 mL) was added NH3.H20 (30 mL, 33% aqueous solution) in one portion at 150 °C under N2. The mixture was stirred at 150 °C for 10 hours. The reaction mixture was diluted with H20 (500 mL) and extracted with EtOAc (500 mL x 3). The combined organic layers were washed withbrine (500 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to givea residue. The residue was purified by column chromatography (Si02, Petroleum ether/Ethylacetate=1:2) to give compound 51.7 (35 g, 95% yield) as a white solid. LCMS (ESI) m/z: [M+ H] calcd for C9H8BrN2: 223; found 223; RT=0.761 mm.
References:
CORTEXYME, INC.;LYNCH, Casey C.;KONRADI, Andrei;GALEMMO, JR., Robert A. WO2018/209132, 2018, A1 Location in patent:Paragraph 0319; 0407; 0431; 0436; 0443
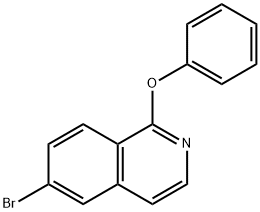
215453-25-1
8 suppliers
inquiry

215453-26-2
110 suppliers
$50.00/25 mg
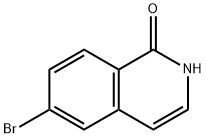
82827-09-6
154 suppliers
$19.00/250mg

215453-26-2
110 suppliers
$50.00/25 mg
34784-05-9
318 suppliers
$5.00/1g

215453-26-2
110 suppliers
$50.00/25 mg