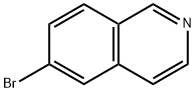
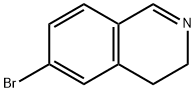
6-Bromoisoquinoline synthesis
- Product Name:6-Bromoisoquinoline
- CAS Number:34784-05-9
- Molecular formula:C9H6BrN
- Molecular Weight:208.05
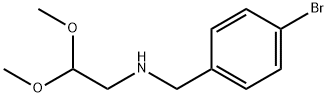
6-Bromoisoquinoline can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly in laboratory organic synthesis and chemical and pharmaceutical research and development process. It can be synthesized by N-(4-bromobenzyl)-2,2-dimethoxyethanamine and Methanesulfonyl chloride react via ring-closure reaction.
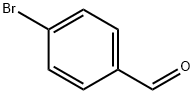
1122-91-4
673 suppliers
$9.00/10g
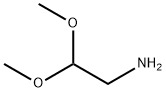
22483-09-6
476 suppliers
$5.00/5g

34784-05-9
319 suppliers
$5.00/1g
Yield:34784-05-9 35%
Reaction Conditions:
Stage #1:4-bromo-benzaldehyde;2,2-dimethoxyethylamine in toluene for 12 h;Reflux;Dean-Stark;
Stage #2: with chloroformic acid ethyl ester in tetrahydrofuran at -10; for 0.166667 h;Further stages;
Steps:
1 Synthesis of 6-bromoisoquinoline (A1).
A mixture of 4-bromobenzaldehyde (300.0 g, 1620.0 mmol) and amino acetaldehyde dimethyl acetal (170.4 g, 1620 mmol) in anhydrous toluene (1.5 L) was refluxed under a Dean-Stark condenser for 12 h. The solution was concentrated under vacuum. The residue was dissolved in anhydrous THF and cooled to -10 °C. Ethyl chloroformate (193.3 ml_, 1782 mmol) was added and stirred for 10 min at -10 °C, and then allowed to warm to room temperature. Subsequently trimethyl phosphite (249.6 ml_, 1782.0 mmol) was added dropwise to the reaction mixture and stirred for 10 h at room temperature. The solvent was evaporated under vacuum and the residue was dissolved in anhydrous DCM (1.5 L) and stirred for 30 minutes. The reaction mixture was cooled to 0 °C, and titanium tetrachloride (1.2 L, 6480 mmol) was added dropwise. The reaction mixture was stirred at 40 °C for 6 days. The reaction mixture was poured into ice and pH was adjusted to 8 - 9 with aqueous 6N NaOH solution. The suspension was extracted three times with EtOAc. The organic layer was extracted with 3 M HCI. The acidic aqueous solution was adjusted to pH to 7 - 8 with 3N NaOH solutions and extracted two times with EtOAc. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to provide the product. Crude compound was dissolved in minimum amount of DCM and mixed with pentane to get compound A1 as light brown solid. Yield: 90 g (35%). Rf: 0.6 (30% EtOAc in petroleum ether). LCMS m/z = 209 (M + 1 ). 1H NMR (400 MHz, cf6-DMSO): δ 7.82 (m, 2H), 8.1 1 (d, J = 8.8 Hz, 2H), 8.30 (br s, 1 H), 8.56 (d, J = 6.0 Hz, 1 H), 9.35 (s, 1 H).
References:
PFIZER INC.;CHEKLER, Eugene Lvovich Piatnitski;GILBERT, Adam Matthew;UNWALLA, Rayomand Jal;VERHOEST, Patrick Robert;ANDERSON, James Thomas WO2015/181676, 2015, A1 Location in patent:Page/Page column 132-133

33065-61-1
79 suppliers
$62.00/250mg

34784-05-9
319 suppliers
$5.00/1g
1036378-91-2
6 suppliers
inquiry

34784-05-9
319 suppliers
$5.00/1g
1036378-89-8
14 suppliers
inquiry

34784-05-9
319 suppliers
$5.00/1g
226942-29-6
155 suppliers
$19.00/250mg

34784-05-9
319 suppliers
$5.00/1g
1393714-90-3
1 suppliers
inquiry