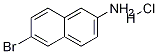
6-Bromonaphthalen-2-amine hydrochloride synthesis
- Product Name:6-Bromonaphthalen-2-amine hydrochloride
- CAS Number:71590-31-3
- Molecular formula:C10H9BrClN
- Molecular Weight:258.5421
5773-80-8
300 suppliers
$10.00/1g

71590-31-3
67 suppliers
$9.00/250mg
Yield:71590-31-3 91%
Reaction Conditions:
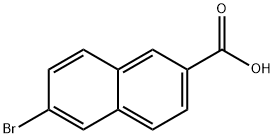
Stage #1: 6-bromo-2-naphthoic acidwith diphenyl phosphoryl azide;triethylamine in tert-butyl alcohol; for 15 h;Reflux;
Stage #2: with hydrogenchloride in methanol at -78 - 20; for 15 h;
Steps:
6.A
A mixture of 6-bromo-2-naphthoic acid (80.3 g, 319 mmol), diphenylphosphoryL azide (71 mL, 352 mmol) and triethylamine (50 mL, 358 mmol) in fert-butanol (400 mL) was heated to reflux and allowed to stir at this temperature for 15 hours. The reaction mixture was then cooled to room temperature and poured over saturated aqueous NaHC03 solution (6Θ0 mL) and stirred vigorouslyfor 30 minutes. The resulting suspension was filtered, washed with water (200 mL) and dried in vacuo at 65 °C. The resulting white solid was suspended in MeOH (500 mL) and cooled to -78 °C, then HCl gas was bubbled into the mixture until saturated. The reaction mixture was then allowed to stir at room -temperature for 15 hours, after which time the resulting solids were collected by filtration, then washed with- ice-cold MeOH (100 mL) to provide Compound Int-6a as an off-white solid (74.8 g, 91%), which was used without further purification. l NM (DMSO-_?) 6 10.5-10.0 (br s, 3H), 8.23 (s, 1H), 7.99 (d, J= 9.0 Hz, 1H), 7.92 (d, J= 9.0 Hz, 1H), 7.84 (s, 1H), 7.68-7.65 (m, 1H), 7.56-7.51 (m, 1H . "LRMS: (M+2H)+ = 223.
References:
WO2011/87740,2011,A1 Location in patent:Page/Page column 74

869114-68-1
42 suppliers
$205.00/1g

71590-31-3
67 suppliers
$9.00/250mg