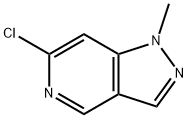
6-Chloro-1-methyl-1H-pyrazolo[4,3-c]pyridine synthesis
- Product Name:6-Chloro-1-methyl-1H-pyrazolo[4,3-c]pyridine
- CAS Number:1558302-68-3
- Molecular formula:C7H6ClN3
- Molecular Weight:167.6
![6-Chloro-1H-pyrazolo[4,3-c]pyridine](/CAS/GIF/1206979-33-0.gif)
1206979-33-0
162 suppliers
$17.00/250mg

74-88-4
342 suppliers
$15.00/10g
![6-Chloro-1-methyl-1H-pyrazolo[4,3-c]pyridine](/CAS/20180703/GIF/1558302-68-3.gif)
1558302-68-3
34 suppliers
inquiry
Yield:1558302-68-3 63.2%
Reaction Conditions:
Stage #1: 6-chloro-1H-pyrazolo[4,3-c]pyridinewith sodium hydride in N,N-dimethyl-formamide at 20; for 0.0333333 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20; for 0.5 h;
Steps:
599.1 Step 1. Synthesis of 6-chloro-1-methyl-1H-pyrazolo [4, 3-c] pyridine
To a solution of 6-chloro-1H-pyrazolo [4, 3-c] pyridine (600 mg, 3.91 mmol) in DMF (10 mL) was added NaH (312.56 mg, 7.81 mmol, 60%purity) at rt, the reaction mixture was stirred at rt for 2 min, then MeI (1.11 g, 7.81 mmol) was added dropwise to above mixture and stirred for 30 min. The reaction mixture was poured into water and extracted with EtOAc. The organic layers were combined and washed with brined, dried over Na2SO4, filtered, and concentrated. The resulting residue was purified by silica gel flash chromatography (petroleum ether/EtOAc) to provide the title compound (414 mg, 63.2%yield) as a white solid. MS (ESI) m/z = 168.2 [M+H]+.
References:
WO2022/73469,2022,A1 Location in patent:Paragraph 2794-2796
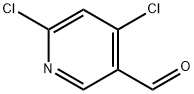
1060811-62-2
133 suppliers
$15.00/100mg
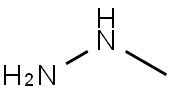
60-34-4
2 suppliers
$65.59/25g
![6-Chloro-1-methyl-1H-pyrazolo[4,3-c]pyridine](/CAS/20180703/GIF/1558302-68-3.gif)
1558302-68-3
34 suppliers
inquiry
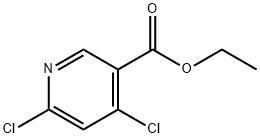
40296-46-6
327 suppliers
$6.00/1g
![6-Chloro-1-methyl-1H-pyrazolo[4,3-c]pyridine](/CAS/20180703/GIF/1558302-68-3.gif)
1558302-68-3
34 suppliers
inquiry