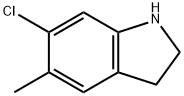
6-Chloro-5-methylindoline synthesis
- Product Name:6-Chloro-5-methylindoline
- CAS Number:162100-44-9
- Molecular formula:C9H10ClN
- Molecular Weight:167.64
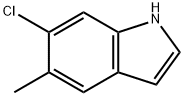
162100-42-7
40 suppliers
$60.00/2mg

162100-44-9
22 suppliers
inquiry
Yield:162100-44-9 3.5 g
Reaction Conditions:
with sodium cyanoborohydride;acetic acid at 20; for 1 h;Inert atmosphere;Industrial scale;
Steps:
1.4 Step 4:
To a solution of Compound D (3.5 g, 21 mmol) in AcOH (40 mL) was added sodium cyanoborohydride (2.7 g, 43 mmol) and the mixture was purged with N2 for 1 h at room temperature.The reaction was complete by TLC (PE / EA = 10/1), further ethyl acetate EA (200 ml) was added and the mixture was made basic with a 5N NaOH solution (200 ml) at a pH of about 8. The organic layer was separated, Wash once, fullAnd brine once, dried over anhydrous sodium sulfate, dried to give 3.5g of brown solid Compound E, spare. Yield 98%.
References:
CN106278987,2017,A Location in patent:Paragraph 0033; 0038

71270-61-6
11 suppliers
$181.00/500mg

162100-44-9
22 suppliers
inquiry
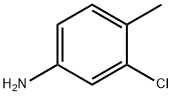
95-74-9
235 suppliers
$9.00/25g

162100-44-9
22 suppliers
inquiry

755031-80-2
24 suppliers
inquiry

162100-44-9
22 suppliers
inquiry