
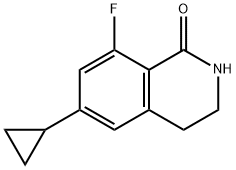
6-Cyclopropyl-8-fluoroisoquinolin-1(2H)-one synthesis
- Product Name:6-Cyclopropyl-8-fluoroisoquinolin-1(2H)-one
- CAS Number:1242156-53-1
- Molecular formula:C12H10FNO
- Molecular Weight:203.21
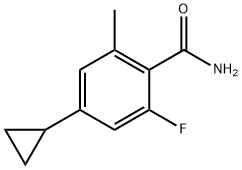
1242156-52-0
24 suppliers
inquiry
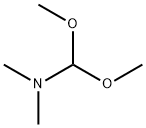
4637-24-5
598 suppliers
$5.00/25g

1242156-53-1
36 suppliers
$307.00/50mg
Yield:1242156-53-1 77%
Reaction Conditions:
Stage #1: 4-cyclopropyl-2-fluoro-6-methylbenzamide;N,N-dimethyl-formamide dimethyl acetal in 2-methyltetrahydrofuran at 60;
Stage #2: with potassium tert-butylate in tetrahydrofuran;2-methyltetrahydrofuran at 55 - 60;
Steps:
3 6-cyclopropyl-8-fluoroisoquinolin-1(2H)-one
To a solution of 4-cyclopropyl-2-fluoro-6-methylbenzamide (37.2 g, 0.19 mol) in 2-methyltetrahydrofuran (MeTHF; 223 ml) was added 1,1-dimethoxy-N,N-dimethylmethanamine (29.8 g, 0.25 mol). The mixture was heated to 60° C. for 2 hours, then around 100 mL of MeTHF was distilled out under vacuum in order to remove Methanol. The reaction mixture was heated to 55° C. again, and potassium tert-butoxide, 1 M solution in THF (289 ml, 0.29 mol) was added dropwise. After 1 hr stirring at 60° C., the reaction mixture was allowed to cool down to room temperature and poured into HCl, 1 M solution (289 ml, 0.29 mol), and then THF/MeTHF was distilled out at 60° C. for crystallization. During the distillation, IPA (223 ml) was added slowly. After most of THF/MeTHF was removed, the solution was cooled down to ambient temperature. The desired product was crystallized out from IPA/water, collected by filtration and washed with water and cold IPA. The filter cake was dried under vacuum at 50° C. to afford 30.1 g of the title compound (77% isolated yield) as a white solid. MS (ESI) 204 (M+H)+.
References:
US2010/222325,2010,A1 Location in patent:Page/Page column 58-59
1242157-16-9
6 suppliers
inquiry

1242156-53-1
36 suppliers
$307.00/50mg
1242157-23-8
86 suppliers
$45.00/50mg

1242156-53-1
36 suppliers
$307.00/50mg

1242156-51-9
25 suppliers
inquiry

1242156-53-1
36 suppliers
$307.00/50mg

1242156-52-0
24 suppliers
inquiry

1242156-53-1
36 suppliers
$307.00/50mg