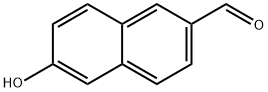
6-Hydroxy-2-naphthaldehyde synthesis
- Product Name:6-Hydroxy-2-naphthaldehyde
- CAS Number:78119-82-1
- Molecular formula:C11H8O2
- Molecular Weight:172.18
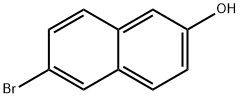
15231-91-1
406 suppliers
$7.00/5g

78119-82-1
186 suppliers
$20.00/1g
Yield:78119-82-1 78.6%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;N,N-dimethyl-formamide at -78 - 20; for 2 h;Inert atmosphere;
Steps:
2.h (H) Preparation of 6-hydroxy-2-naphthaldehyde
6-bromo-2-naphthol (0.91 g, 4.1 mmol) was dissolved in 20 mL of dry THF, protected under nitrogen, and n-BuLi (3.4 mL, 8.2 mmol) was added, stirred for 1 hour and then anhydrous DMF (0.941 mL, 12.3 mmol) was added dropwise at -78 ° C,The reaction was continued for 1 hour, raised to room temperature, acidified with 2N hydrochloric acid to pH = 5, extracted with ethyl acetate, washed with water, washed with saturated brine,Dried over anhydrous sodium sulfate, filtered and concentrated to give hexanesulfonic acid (8: 1) column chromatography to give 6-hydroxy-2-naphthaldehyde, yield 78.6%
References:
Liaocheng University;Liu Guoyun;Yang Jie;Li Xiaoteng;Guo Shangjing CN105111054, 2017, B Location in patent:Paragraph 0088; 0089; 0090
309752-65-6
39 suppliers
$204.00/1g

78119-82-1
186 suppliers
$20.00/1g
3453-33-6
324 suppliers
$31.00/5g

78119-82-1
186 suppliers
$20.00/1g
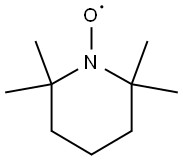
2564-83-2
572 suppliers
$6.00/10g

309752-65-6
39 suppliers
$204.00/1g

78119-82-1
186 suppliers
$20.00/1g
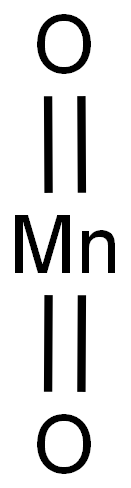
1313-13-9
479 suppliers
$13.00/5g

309752-65-6
39 suppliers
$204.00/1g

78119-82-1
186 suppliers
$20.00/1g