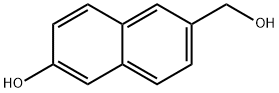
6-(Hydroxymethyl)-2-naphthol synthesis
- Product Name:6-(Hydroxymethyl)-2-naphthol
- CAS Number:309752-65-6
- Molecular formula:C11H10O2
- Molecular Weight:174.2
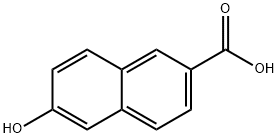
16712-64-4
347 suppliers
$5.00/5g

309752-65-6
38 suppliers
$204.00/1g
Yield:309752-65-6 68.53%
Reaction Conditions:
with sodium borohydrid;dimethyl sulfate in tetrahydrofuran;water
Steps:
7 Example 7
Example 7 Under a nitrogen atmosphere, 3.90 g (101 mmol) of sodium borohydride (purity: 98%) and 60 mL of anhydrous tetrahydrofuran were introduced into a 300-mL four-neck flask equipped with a dropping funnel, a gas inlet pipe, a condenser and a thermometer and stirred. The mixture was added dropwise with 13.21 g (105 mmol) of dimethyl sulfate at 0°C over 5 minutes and stirred for 2 hours with ice cooling. The mixture was further added with 40 mL of anhydrous tetrahydrofuran and stirred at room temperature for 3 hours, and the stop of gas generation was observed. Subsequently, the reaction mixture was added with a solution of 9.73 g (51.71 mmol) of 6-hydroxy-2-naphthalenecarboxylic acid in anhydrous tetrahydrofuran (50mL) at room temperature over 25 minutes by using a dropping funnel. The reaction mixture solidified during the addition. This reaction mixture was stirred at the same temperature for 7 hours. After the reaction was completed, the reaction mixture was cooled, added with 150 mL of water with ice cooling and vigorously stirred at room temperature for 30 minutes. The resulting reaction mixture was analyzed by liquid chromatography using the internal standard method. As a result, it was found that the conversion of the 6-hydroxy-2-naphthalenecarboxylic acid was 68.53% and the yield of the 6-hydroxy-2-naphthylcarbinol was 26.8%.
References:
MITSUBISHI CHEMICAL CORPORATION EP1186588, 2002, A1
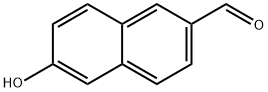
78119-82-1
181 suppliers
$20.00/1g

309752-65-6
38 suppliers
$204.00/1g
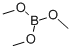
121-43-7
345 suppliers
$13.00/25mL

16712-64-4
347 suppliers
$5.00/5g

309752-65-6
38 suppliers
$204.00/1g
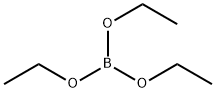
150-46-9
186 suppliers
$12.00/25mL

16712-64-4
347 suppliers
$5.00/5g

309752-65-6
38 suppliers
$204.00/1g
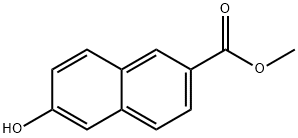
17295-11-3
96 suppliers
$7.00/250mg

309752-65-6
38 suppliers
$204.00/1g