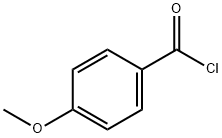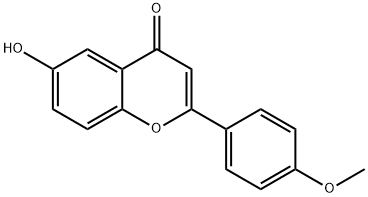
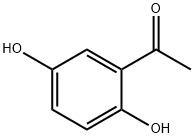
6-HYDROXY-4''-METHOXYFLAVONE synthesis
- Product Name:6-HYDROXY-4''-METHOXYFLAVONE
- CAS Number:35794-88-8
- Molecular formula:C16H12O4
- Molecular Weight:268.26
Yield:35794-88-8 70%
Reaction Conditions:
Stage #1: 2,5-Dihydroxyacetophenonewith pyridine;water;potassium carbonate in acetone at 60; for 0.25 h;
Stage #2: 4-methoxy-benzoyl chloride in acetone;Reflux;
Steps:
General procedure for preparation of flavones16a-16e, 19a-19e, 22a-22e, 25a-25d.
To a solutionof acetophenone (1 mmol) in wet acetone (containing1% w/w water) (20 mL/mmol) was added potassiumcarbonate (8 equiv.) and then pyridine (4 equiv.). Thesolution was stirred at 60°C for 15 min and aroylchloride (2 equiv.) was slowly added. The reactionmixture was stirred upon refluxing for 24-48 h. Aftercooling down to room temperature, acetone wasevaporated. Acetic acid (10%) was added, the solutionwas stirred till it stopped bubbling. The residue wasfiltered off, washed with water (2×50 mL) to make itneutral, vacuum-dried, and then crystallized fromacetone (20 mL) to produce pure products.
References:
Wang;Liu;Zhang [Russian Journal of General Chemistry,2018,vol. 88,# 5,p. 1036 - 1041][Zh. Obshch. Khim.,2018,# 5,p. 1036 - 1041,6]
490-78-8
339 suppliers
$5.00/50mg

35794-88-8
5 suppliers
inquiry

457930-31-3
0 suppliers
inquiry

35794-88-8
5 suppliers
inquiry