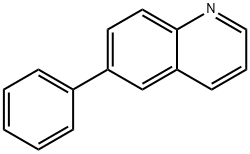
6-phenylquinoline synthesis
- Product Name:6-phenylquinoline
- CAS Number:612-95-3
- Molecular formula:C15H11N
- Molecular Weight:205.25
Yield:612-95-3 82%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in ethanol;water;toluene at 95; for 16 h;
Steps:
6
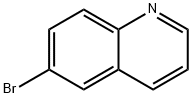
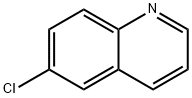
A vessel was charged with 6-bromoquinoline (2.08 g, 10 mmol), and ethanol (10 mL), water (20 mL), toluene (40 mL), phenylboronic acid (1.83 g, 15 mmol, 1.5 equiv), K2C03 (5.52 g, 40 mmol, 4.0 equiv), and Pd(PPh3)4 (1.15 g, 1 mmol, 0.1 equiv) were added. The resulting mixture was heated at 95°C for 16 hours. After cooling to room temperature, the biphasic solution was diluted with saturated aqueous NH4C1 (30 mL) and CH2C12 (30 mL). The aqueous phase was extracted with CH2C12 (2x30 mL) and the combined organic layers were washed with water (30 mL) and saturated aqueous NaHCO3 (30 mL). The organic phase was dried over MgSO4 and filtered. The filtrate was concentrated in vacuo and purified by flash column chromatography to afford 6-phenylquinoline (1.68 g, 82 %).
References:
WO2014/43243,2014,A2 Location in patent:Paragraph 00232