
L-Alanine, N-[(1,1-diMethylethoxy)carbonyl]-3-[[(phenylMethoxy)carbonyl]aMino]-, Methyl ester synthesis
- Product Name:L-Alanine, N-[(1,1-diMethylethoxy)carbonyl]-3-[[(phenylMethoxy)carbonyl]aMino]-, Methyl ester
- CAS Number:61040-22-0
- Molecular formula:C17H24N2O6
- Molecular Weight:352.38
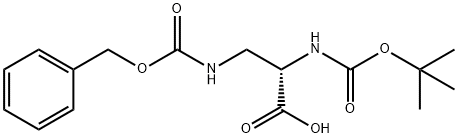
65710-57-8
155 suppliers
$5.00/250mg

74-88-4
357 suppliers
$15.00/10g
![L-Alanine, N-[(1,1-diMethylethoxy)carbonyl]-3-[[(phenylMethoxy)carbonyl]aMino]-, Methyl ester](/CAS/GIF/61040-22-0.gif)
61040-22-0
9 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 20; for 12 h;
Steps:
4.15.1 Step 1 :
A mixture of 3-{[(benzyloxy)carbonyl]amino}-N-(tert- butoxycarbonyl)alanine (100 g, 0.29 mol), potassium carbonate (82 g, 0.59 mol) and methyl iodide (42 g, 0.29 mol) in DMSO (1200 mL) was stirred at room temperature. After 12 hours, the reaction mixture was extracted with ethyl acetate, and the organics were separated and concentrated under reduced pressure. The residue was purified by chromatography on silica gel to give methyl 3-{[(benzyloxy)carbonyl]amino}-N-(tert-butoxycarbonyl)alaninate. MS ESI calc'd. for C17H25N2O6 [M + H]+ 353, found 353. 1H NMR (400 MHz, CDCI3) δ 7.36 - 7.33 (m, 5H), 5.09 (s, 2H), 4.34 - 4.30 (m, 1H), 3.74 (s, 2H), 3.59 (t, J= 6.0 Hz, 2H), 2.62 (s, 3H), 1.44 (s, 9H).
References:
WO2013/52394,2013,A1 Location in patent:Paragraph 00317
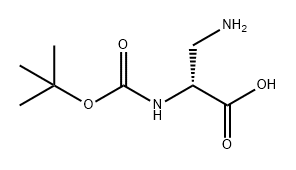
76387-70-7
176 suppliers
$9.00/100mg
![L-Alanine, N-[(1,1-diMethylethoxy)carbonyl]-3-[[(phenylMethoxy)carbonyl]aMino]-, Methyl ester](/CAS/GIF/61040-22-0.gif)
61040-22-0
9 suppliers
inquiry
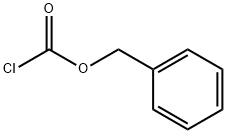
501-53-1
409 suppliers
$10.00/1g
![L-Alanine, N-[(1,1-diMethylethoxy)carbonyl]-3-[[(phenylMethoxy)carbonyl]aMino]-, Methyl ester](/CAS/GIF/61040-22-0.gif)
61040-22-0
9 suppliers
inquiry