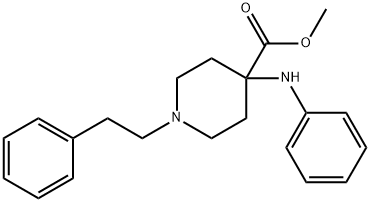
4-Piperidinecarboxylic acid, 4-(phenylamino)-1-(2-phenylethyl)-, methyl ester synthesis
- Product Name:4-Piperidinecarboxylic acid, 4-(phenylamino)-1-(2-phenylethyl)-, methyl ester
- CAS Number:61085-55-0
- Molecular formula:C21H26N2O2
- Molecular Weight:338.44
Yield:61085-55-0 65%
Reaction Conditions:
with trifluorormethanesulfonic acid in dimethyl sulfoxide at 0; under 760.051 Torr; for 96 h;Reflux;
Steps:
Methyl 4-(phenylamino)-1-(2-phenylethyl)-4-piperidinecarboxylate (3)
Methanol (65 ml) was poured into a 250 ml round bottom flask and cooled to 0°C in an ice bath. Trifluorormethanesulfonic acid (11.5 ml) was added dropwise to the MeOH with intensive stirring and cooling (Caution: Very corrosive vapors and liquid Greatly exothermic process). After the addition of CF3SO3H was complete, the ice bath was removed and 2.2 ml DMSO was added, followed by the addition of 3.16 g amide (2).The reaction mixture was refluxed and monitored by TLC (DCM:MeOH, 8:1) until the starting material was mostly consumed (after about 4 days of refluxing), about 90% conversion. The reaction mixture was cooled down to room temperature and slowly poured into 300 ml of 10% aqueous K2CO3. The mixture was transferred to a separatory funnel and the product was extracted with 6 x 50 ml EtOAc. Combined extracts were washed with 2 x 50 ml aq. NaHCO3 solution, followed by drying over anhydrous Na2SO4 and removal of the volatiles under reduced pressure. The obtained crude product was purified by flash chromatography on silica gel with DCM:EtOAc:iPrOH 6:2:1. Methyl 4-(phenylamino)-1-(2-phenylethyl)-4-piperidinecarboxylate (2.09 g) was obtained in 65% yield as a creamy white solid, mp. 92-94°C, lit.6 mp. 94.9 C; Rf=0.5; 1H NMR (700 MHz, CDCl3) d: 2.08 (d, J14.0 Hz, 2H, CH2), 2.30(t, J10.5 Hz, Ph-CH2-CH2-N, 2H), 2.54 (t, J10.5 Hz, Ph-CH2-CH2-N, 2H), 2.64 -2.66 (m, 2H, CH2), 2.73 (s, CH2, 2H), 2.81-2.83 (m, CH2, 2H), 3.69 (s, COOCH3, 3H),3.87 (s, NH, 1H), 6.58 (d, J 8.4 Hz, Ar(H), 2H), 6.76 (t, J 7.7 Hz, Ar(H), 1H), 7.14- 7.16 (m, Ar(H), 2H), 7.19 - 7.21 (m, Ar(H), 3H), 7.25 - 7.29 (m, Ar(H), 2H). 13CNMR (125 MHz, CDCl3) d: 32.86, 33.56, 48.99, 52.36, 58.17, 60.30, 115.51, 118.87,126.16, 128.45, 128.69, 129.17, 139.99, 144.85, 175.68.
References:
Vellem?e, Eerold;Mastitski, Anton;J?rv, Jaak;Veli Hiltunen, Jukka [Organic Preparations and Procedures International,2018,vol. 50,# 5,p. 522 - 526]
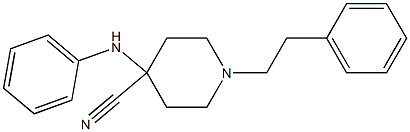
61085-36-7
1 suppliers
inquiry

61085-55-0
2 suppliers
inquiry
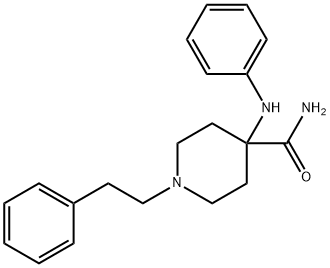
61085-40-3
0 suppliers
inquiry

61085-55-0
2 suppliers
inquiry

62-53-3
636 suppliers
$10.00/1g

61085-55-0
2 suppliers
inquiry
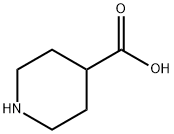
498-94-2
1 suppliers
$5.00/10g

61085-55-0
2 suppliers
inquiry
