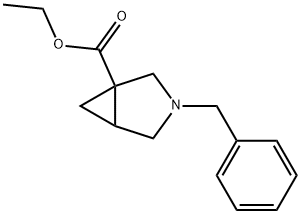
3-Azabicyclo[3.1.0] hexane-1-carboxylic acid, 3-(phenylMethyl)-, ethyl ester synthesis
- Product Name:3-Azabicyclo[3.1.0] hexane-1-carboxylic acid, 3-(phenylMethyl)-, ethyl ester
- CAS Number:63618-07-5
- Molecular formula:C15H19NO2
- Molecular Weight:245.32
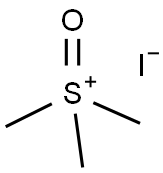
1774-47-6
538 suppliers
$6.00/25g
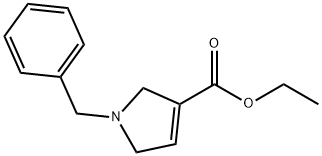
128259-46-1
4 suppliers
inquiry
![3-Azabicyclo[3.1.0] hexane-1-carboxylic acid, 3-(phenylMethyl)-, ethyl ester](/CAS/GIF/63618-07-5.gif)
63618-07-5
41 suppliers
$63.00/100mg
Yield:63618-07-5 43%
Reaction Conditions:
with sodium hydride in dimethyl sulfoxide at 20;
Steps:
35
[ -(3-azabicyclo[3.1.0]hexan-l -yl)-3-methyl-l ,2,4-oxadiazole[00376] The N-benzyl-2,5-dihydropyrrole ester was prepared, as described (Chem. Pharm. Bull. 1985, 33(7), 2762) by treating ethyl propiolate and N-(methoxymethyl)-N- (trimethylsilylmethyl)-N-benzylamine in DCM with 0.1M TFA in DCM, followed by aqueous work up and silica gel chromatography, in 44% yield. The cyclopropyl group was furnished by treatment with trimethylsulfoxonium Iodide and NaH in DMSO at room temperature, followed by aqueous work up and silica gel chromatography, in 43% yield as described in the literature (Korean J. of Med. Chem. 1994, 4(2), 1 19). The N-benzyl group was removed with palladium on carbon and ammonium formate in methanol. The Boc protecting group was directly installed with DMAP, TEA and Boc20 in DCM, followed by silica gel chromatography, in 60% yield for both steps. The oxadiazole ring was prepared as described above, by reaction with acetamide oxime and sodium methoxide in methyl THF. Aqueous work up and silica gel chromatography provided the intermediate in 54% yield. Standard deblocking with HCl-EtOH provided the desired product 56, as the HCI salt, in 82% yield.
References:
WO2011/85406,2011,A1 Location in patent:Page/Page column 150
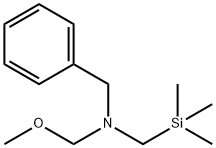
93102-05-7
295 suppliers
$6.00/1g
![3-Azabicyclo[3.1.0] hexane-1-carboxylic acid, 3-(phenylMethyl)-, ethyl ester](/CAS/GIF/63618-07-5.gif)
63618-07-5
41 suppliers
$63.00/100mg

623-47-2
332 suppliers
$10.00/1g
![3-Azabicyclo[3.1.0] hexane-1-carboxylic acid, 3-(phenylMethyl)-, ethyl ester](/CAS/GIF/63618-07-5.gif)
63618-07-5
41 suppliers
$63.00/100mg

93102-05-7
295 suppliers
$6.00/1g

623-47-2
332 suppliers
$10.00/1g
![3-Azabicyclo[3.1.0] hexane-1-carboxylic acid, 3-(phenylMethyl)-, ethyl ester](/CAS/GIF/63618-07-5.gif)
63618-07-5
41 suppliers
$63.00/100mg