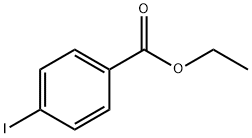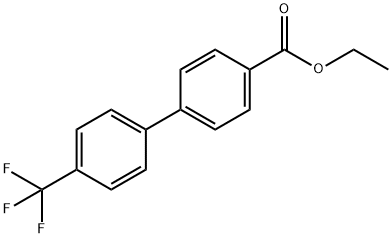
[1,1'-Biphenyl]-4-carboxylic acid, 4'-(trifluoromethyl)-, ethyl ester synthesis
- Product Name:[1,1'-Biphenyl]-4-carboxylic acid, 4'-(trifluoromethyl)-, ethyl ester
- CAS Number:647842-34-0
- Molecular formula:C16H13F3O2
- Molecular Weight:294.27
Yield:647842-34-0 73%
Reaction Conditions:
Stage #1: Ethyl 4-bromobenzoatewith (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;2,2'-bis(1,3,2-benzodioxaborole);potassium acetate in ethanol;ethylene glycol at 80; for 2.5 h;Inert atmosphere;
Stage #2: p-trifluoromethylphenyl bromidewith potassium phosphate tribasic trihydrate in ethanol;ethylene glycol;dimethyl sulfoxide at 80; for 18 h;
Steps:
14
In a reaction vessel substituted with argon2,2'-bi (1,3,2-benzodioxabolol) (1.10 mmol, 262 mg), dichloro [1,1'-bis (diphenylphosphino) ferrocene] palladium (0.030 mmol, 25 mg) , Potassium acetate (3.00 mmol, 294 mg),Ethanol (6.0 mL) and ethyl 4-bromobenzoate (1.00 mmol (229 mg) was added, and the mixture was stirred at 80 ° C. for 2 hours. 1,2-Ethylenediol (2.20 mmol, 137 mg) was added to the obtained reaction solution, and the mixture was stirred at 80 ° C. for 30 minutes.did. Then potassium phosphate trihydrate (3.00 mmol, 798 mg), 4-bromobenzotrifluoride (1.00 mmol, 225 mg) and dimethyl sulfoxide (2)... 0 mL) was added, and the mixture was further stirred at 80 ° C. for 18 hours. After completion of the reaction, water and hexane were added to the reaction mixture, and the product was extracted into a hexane layer. The hexane layer was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated. The obtained residue is refined by silica gel chromatography.A colorless and transparent liquid of ethyl 4'-trifluoromethyl-1,1'-biphenyl-4-carboxylate was obtained (215 mg, yield 73%).
References:
JP5770503,2015,B2 Location in patent:Paragraph 0071-0072

5798-75-4
187 suppliers
$6.00/5g
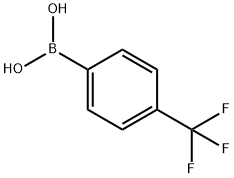
128796-39-4
424 suppliers
$10.00/5g
![[1,1'-Biphenyl]-4-carboxylic acid, 4'-(trifluoromethyl)-, ethyl ester](/CAS/GIF/647842-34-0.gif)
647842-34-0
3 suppliers
inquiry

5798-75-4
187 suppliers
$6.00/5g
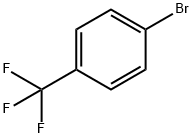
402-43-7
428 suppliers
$6.00/10g
581-80-6
32 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid, 4'-(trifluoromethyl)-, ethyl ester](/CAS/GIF/647842-34-0.gif)
647842-34-0
3 suppliers
inquiry