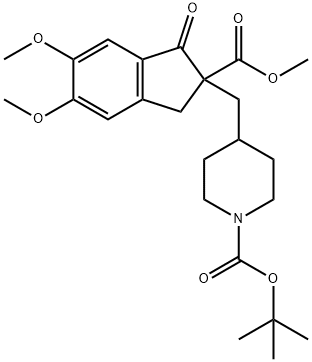
1-t-BOC-[4-((5,6-diMethoxy-2-Methoxycarbonylindan-1-on)-2yl) Methyl]piperidine synthesis
- Product Name:1-t-BOC-[4-((5,6-diMethoxy-2-Methoxycarbonylindan-1-on)-2yl) Methyl]piperidine
- CAS Number:652130-41-1
- Molecular formula:C24H33NO7
- Molecular Weight:447.52

119035-03-9
10 suppliers
inquiry
145508-94-7
90 suppliers
$5.00/100mg
![1-t-BOC-[4-((5,6-diMethoxy-2-Methoxycarbonylindan-1-on)-2yl)
Methyl]piperidine](/CAS/GIF/652130-41-1.gif)
652130-41-1
14 suppliers
inquiry
Yield:652130-41-1 49.9%
Reaction Conditions:
Stage #1: 5,6-dimethoxy-2-methoxycarbonylindan-1-onewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.5 h;
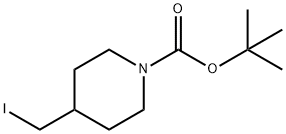
Stage #2: tert-butyl 4-(iodomethyl)piperidine-1-carboxylate in DMF (N,N-dimethyl-formamide) at 20 - 50; for 5.5 h;
Steps:
2 1-t-BOC-[4-((5,6-dimethoxy-2-methoxycarbonylindan-1-on)-2yl)methyl]piperidine:
Example 2 1-t-BOC-[4-((5,6-dimethoxy-2-methoxycarbonylindan-1-on)-2yl)methyl]piperidine: 5,6-dimethoxy-2-methoxycarbonylindan-1-one (1.41 g) was dissolved in DMF (30 mL). Potassium carbonate (1.56 g) was added and the mixture was stirred for 30 min at room temperature. A solution of 1-t-BOC-piperidyl-4-methyliodide (2.2 g) dissolved in 10 mL DMF was added dropwise within 30 min. The mixture was heated gently to (45-50° C.) and the reaction mixture was stirred at 50° C. for 5 hrs. Ethyl acetate (30 mL) was added to the reaction mixture, and the solution was washed with water (3*30 mL). The organic layer was separated, dried over MgSO4 and evaporated to give yellowish solid. The oil obtained was crystallized from ethyl acetate/n-hexane. 1.26 g of the title product (49.9% yield) were obtained as yellow crystals. Mass spectrum m/e: 448[M+H+]. 1H NMR(CDCl3, δ(ppm)): 7.16(s, 1H), 6.89(s, 1H), 3.99(s, 3H), 3.90(s, 3H), 3.69(s, 3H).
References:
US2004/48893,2004,A1 Location in patent:Page/Page column 6