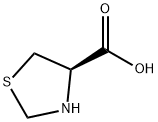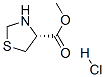
methyl (R)-thiazolidine-4-carboxylate hydrochloride synthesis
- Product Name:methyl (R)-thiazolidine-4-carboxylate hydrochloride
- CAS Number:65983-36-0
- Molecular formula:C5H10ClNO2S
- Molecular Weight:183.6564
Yield:65983-36-0 100%
Reaction Conditions:
with thionyl chloride at 0;
Steps:
27
Compound 261a:[337] The commercial]y available starting material, thiazolidine-4-carboxylic acid (1.3g, 9.59 mmol) was dissolved in anhydrous methanol (19.18 mL) and cooled to 00C in an ice bath. Thionyl chloride (1.40 mL, 19.18 mmol) was added drop wise and the reaction stirred for 30 minutes. The ice bath was removed and stirring continued either for 4-5 hours or overnight. The solvent was stripped and the product placed on the high vacuum to give 4- (methoxycarbonyl)thiazolidin-3-ium chloride. Without further purification and assuming 100% yield, the 4-(methoxycarbonyl)thiazolidin-3-ium chloride (1.761 g, 9.59 mmol) and compound 4 (3.39 g, 10.55 mmol) were each dissolved separately in tetrahydrofuran (32.0 mL) and cooled to 00C. Triethylamine (4.41 mL, 31.6 mmol) was added to the solution with 4- (methoxycarbonyl)thiazolidin-3-ium chloride and then compound 4 was added quickly via canula. After 20 minutes, the pH of the solution was checked to ensure it was basic. The reaction stirred at 00C for 1.5 hours and then at room temperature for 30 minutes and was checked by MS. It was quenched with cold 5% hydrochloric acid and diluted with cold ethyl acetate and water. The solution was extracted with ethyl acetate three times and the combined organic washed with brine, saturated sodium bicarbonate and then brine again. It was dried over sodium sulfate, filtered and stripped. The crude material was purified by silica gel chromatography using a gradient of 50% to 75% ethyl acetate in hexanes to give compound 261a (4.1g, yield = 99%). 1H NMR (400 MHz, CDCl3): the compound appears as a pair of distinct rotomers. δ 7.78 (s, 0.6H), 7.74 (s, 0.4H), 7.48-7.35 (m, 5H), 6.96 (s, 0.4H), 6.92 (s, 0.6H), 5.40 (dd, Ji = 7.0Hz, J2 = 3.4 MHz, 0.6H), 5.31-5.22 (m, 2H), 5.13 (d, 9.6Hz, 0.4H), 4.60 (d, J = 9.6 MHz, 0.4H), 4.46 (dd, Ji = 4.4Hz, J2 = 3.2 MHz, 0.4 H), 4.36 (d, J = 8.4 MHz, 0.6 H), 4.26 (d, J = 8.4Hz, 0.6H), 4.02 (s, 1.8H), 3.96 (s, 1.2 H), 3.86 (s, 1.8H), 3.71 (s, 1.2H), 3.48-3.43 (m, 0.6H), 3.36- 3.29 (m, 1.4H); MS (m/z), found 455.3 (M + Na)+.
References:
WO2010/91150,2010,A1 Location in patent:Page/Page column 210-211