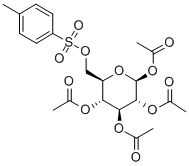
1,2,3,4-TETRA-O-ACETYL-6-O-TOSYL-BETA-D-GLUCOPYRANOSE synthesis
- Product Name:1,2,3,4-TETRA-O-ACETYL-6-O-TOSYL-BETA-D-GLUCOPYRANOSE
- CAS Number:6619-10-9
- Molecular formula:C21H26O12S
- Molecular Weight:502.49

2280-44-6
7 suppliers
inquiry

108-24-7
5 suppliers
$14.00/250ML

98-59-9
585 suppliers
$9.00/5g

6619-10-9
19 suppliers
inquiry
Yield:6619-10-9 39%
Reaction Conditions:
Stage #1: D-Glucose;p-toluenesulfonyl chloridewith pyridine at 0; for 20 h;Inert atmosphere;
Stage #2: acetic anhydride at 0 - 20; for 1 h;Inert atmosphere;
Steps:
1,2,3,4-Tetra-O-acetyl-6-O-p-toluenesulfonyl-β-D-glucopyranose (6-Ts-O-Ac-Glc)
D-Glucose12(500 mg, 2.8 mmol) was added to a solution ofp-toluenesulfonyl chloride (TsCl, 534 mg, 2.8 mmol) in anhydrous pyridine (7.5 mL) at 0°Cunder nitrogen atmosphere and the solution was stirred overnight. Then, Ac2O (2.2 mL) was added dropwise and the solution was allowed to warm to room temperature. After 1h the solvent was removed in reduced pressure and the residue was dissolved in CH2Cl2. The solution was washed several times with saturated NaHCO3aq., 0.2 M HCl aq. and water, dried under Na2SO4, and concentrated. The resulted crude 1,2,3,4-tetra-O-acetyl-6-O-p-toluenesulfonyl-β-D-glucopyranose (6-Ts-O-Ac-Glc) was crystallized from EtOH (0.54 g, 39%) as white solid.
References:
Fadlan, Arif;Tanimoto, Hiroki;Ito, Tatsuya;Aritomi, Yusuke;Ueno, Maho;Tokuda, Masaya;Hirohara, Shiho;Obata, Makoto;Morimoto, Tsumoru;Kakiuchi, Kiyomi [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 8,p. 1848 - 1858] Location in patent:supporting information
37074-90-1
50 suppliers
inquiry

6619-10-9
19 suppliers
inquiry
29907-22-0
0 suppliers
inquiry
563-63-3
265 suppliers
$7.00/1g

64-19-7
1537 suppliers
$10.00/25ML

6619-10-9
19 suppliers
inquiry
