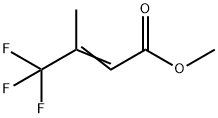
2-Butenoic acid, 4,4,4-trifluoro-3-methyl-, methyl ester synthesis
- Product Name:2-Butenoic acid, 4,4,4-trifluoro-3-methyl-, methyl ester
- CAS Number:671-82-9
- Molecular formula:C6H7F3O2
- Molecular Weight:168.11
Yield:-
Reaction Conditions:
in pentane at -78 - 20;Inert atmosphere;
Steps:
Compound 1.3: Methyl-(4-trifluoro-3-methyl)-but-2-enoate
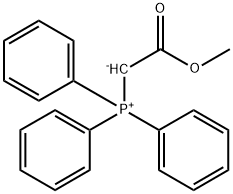
Under an atmosphere of dry argon, 67.0 g (200 mmol) methyl(triphenylphosphoranylidene)acetate were suspended in 300 mL of n-pentane and the mixture was cooled to - 78 00. 33.6 g of trifluoroacetone were added rapidly and stirring at - 78 00 was continued for another three hours. The reaction mixture was allowed to warm to room temperature overnight. Theprecipitate was filtered by frit and the pentane was distilled off using a 20 cm distillation column filled with Raschig rings. The residue was distilled at normal pressure on a rotary band column. There were obtained 87.3 g of pentane and four fractions boiling from 106 - iii 00 containing the target product. The first two of these fractions had assays of 67 % and 85.6 % (GO) and weighed 0.8 and 1 .1 g respectively. The last two fractions contained 97.4 % and 97.9 % oftarget product (GO) and weighed 8.9 and 11.6 g respectively. The product was characterized via its mass spectrum.
References:
WO2017/50609,2017,A1 Location in patent:Page/Page column 18
649-62-7
0 suppliers
inquiry

671-82-9
1 suppliers
inquiry
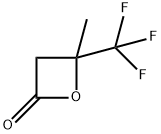
110115-54-3
0 suppliers
inquiry

671-82-9
1 suppliers
inquiry
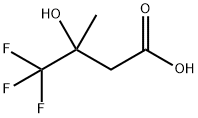
338-03-4
64 suppliers
$15.00/1g

671-82-9
1 suppliers
inquiry