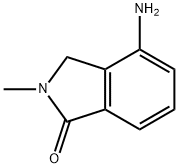
1H-Isoindol-1-one,4-amino-2,3-dihydro-2-methyl-(9CI) synthesis
- Product Name:1H-Isoindol-1-one,4-amino-2,3-dihydro-2-methyl-(9CI)
- CAS Number:682757-53-5
- Molecular formula:C9H10N2O
- Molecular Weight:162.19

682757-52-4
18 suppliers
inquiry

682757-53-5
35 suppliers
$155.00/50mg
Yield:682757-53-5 97%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethyl acetate at 20; for 5.5 h;
Steps:
15.B Step B; Preparation of 4-amino-2,3-dihydro-2-methyl-1H-isoindol-1-one
A slurry of 2, [3-DIHYDRO-2-METHYL-4-NITRO-LH-ISOINDOL-1-ONE] (i. e. product of Step A) (0.97 g, 5.1 mmol) and 10% palladium on carbon (0.24 g) in ethyl acetate (35 mL) was hydrogenated at 45 psi (310 kPa) at room temperature for 5.5 h. The mixture was then filtered through a pad of [CELITE)] diatomaceous filter aid, and the [CELITE (E)] was extracted with ethyl acetate. The filtrate was concentrated under vacuum to give the title compound (0.81 g, 97% yield). 1H NMR [(CDC13)] : [B] 7.29 (m, 2H), 6.80 (d, 1H), 4.21 (s, 2H), 3.20 (s, [3H).]
References:
WO2004/35545,2004,A2 Location in patent:Page 42-43
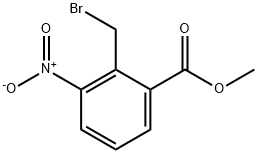
98475-07-1
449 suppliers
$7.00/250mg

682757-53-5
35 suppliers
$155.00/50mg
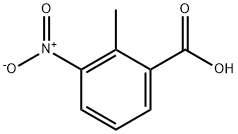
1975-50-4
439 suppliers
$20.00/25g

682757-53-5
35 suppliers
$155.00/50mg
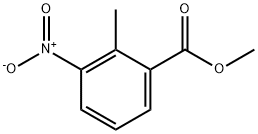
59382-59-1
254 suppliers
$21.00/5g

682757-53-5
35 suppliers
$155.00/50mg