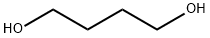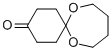
7,12-DIOXASPIRO(5,6)DODECANE-3-ONE synthesis
- Product Name:7,12-DIOXASPIRO(5,6)DODECANE-3-ONE
- CAS Number:80427-20-9
- Molecular formula:C10H16O3
- Molecular Weight:184.23
Yield:80427-20-9 16.74 g (18%)
Reaction Conditions:
with toluene-4-sulfonic acid in toluene;
Steps:
7.A A.
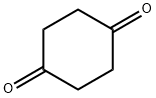
A. 7,12-Dioxaspiro[5.6]dodecan-3-one (Ref.: Hyatt, G. A.; J. Org. Chem., 1983, 48, 129) To a stirred solution of 56.0 g (0.5 mol) of 1,4-cyclohexanedione, and 0.25 g (1.3 mmol) of p-toluenesulfonic acid in 500 ml of toluene under argon, refluxing with a Dean-Stark trap was added 44.5 ml (0.5 mol, 1 eq.) of freshly distilled 1,4-butanediol over 3.5 hours. After refluxing for an additional hour, the mixture was allowed to cool, then washed with 20 ml of saturated NaHCO3, dried over MgSO4 and evaporated. The residue was triturated with an equal volume of EtOAc and cooled in ice. The precipitated bis-ketal was removed by filtration and the solvent was evaporated from the filtrate. Separation of the diketone from the desired ketal was accomplished by three successive distillations, the last two using a 12 inch Vigreux column. This procedure afforded 16.74 g (18%) of title monoketal (b.p. 80°-81° C./0.5 mm) as a colorless, viscous liquid. A trace of cyclohexanedione was present. TLC silica gel (EtOAc), Rf 0.51. 1 H-NMR (CDCl3) δ 3.62 (br s, 4), 2.27 (t, 4H, J=7 Hz), 1.87 (t, 4H, J=7 Hz), 1.53 (m, 4H) ppm.
References:
US4721725,1988,A