
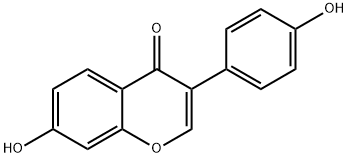
7,4'-Dibenzyl Daidzein synthesis
- Product Name:7,4'-Dibenzyl Daidzein
- CAS Number:1179998-29-8
- Molecular formula:C29H22O4
- Molecular Weight:434.48
486-66-8
575 suppliers
$5.00/1g
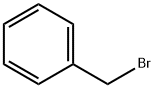
100-39-0
434 suppliers
$10.00/10g

1179998-29-8
9 suppliers
$275.00/50mg
Yield:1179998-29-8 79%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 40; for 2 h;
Steps:
7-Benzyloxy-3-(4-benzyloxyphenyl)chrome-4-one 3
Compound 2 (540.8 mg, 2.11 mmol) was dissolved in dryDMF (2 mL), and K2CO3 (961.8 mg, 6.38 mmol) and benzylbromide (1113.4 mg, 6.38 mmol) were added. The mixture washeated at 40 °C for 2 h. After cooling the mixture to roomtemperature, ethyl acetate and water were added. The organiclayer was washed several times with water and brine, andthen dried over MgSO4. The residue was purified by silicagel column chromatography (eluent; n-hexane/EtOAc = 4 : 1)to afford compound 3 (743.2 mg, 1.70 mmol, 79%) as a yellowsolid. Rf = 0.27 (n-hexane/EtOAc = 4 : 1); 1H-NMR (400 MHz,CDCl3) δ: 8.22 (1H, d, J = 9.2 Hz), 7.91 (1H, s), 7.50-7.33 (10H, m), 7.07-7.03 (3H, m), 6.93 (1H, s), 5.18 (2H, s), 5.11 (2H,s); 13C-NMR (100 MHz, CDCl3) δ: 175.8, 162.9, 152.1, 135.7,130.1, 128.8, 128.6, 128.4, 127.9, 127.5, 127.4, 115.0, 114.9,105.0, 101.3, 70.5, 70.1.
References:
Cho, Hye Won;Gim, Hyo Jin;Li, Hua;Subedi, Lalita;Kim, Sun Yeou;Ryu, Jae-Ha;Jeon, Raok [Chemical and Pharmaceutical Bulletin,2021,vol. 69,# 1,p. 99 - 105]
108-46-3
772 suppliers
$10.00/10g

1179998-29-8
9 suppliers
$275.00/50mg
156-38-7
651 suppliers
$6.00/25g

1179998-29-8
9 suppliers
$275.00/50mg

17720-60-4
66 suppliers
$16.00/100mg

1179998-29-8
9 suppliers
$275.00/50mg
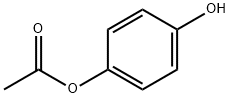
3233-32-7
104 suppliers
$27.00/1g

1179998-29-8
9 suppliers
$275.00/50mg