
7,8,9,10-TETRAHYDRO-BENZO[A]PYREN-7-OL synthesis
- Product Name:7,8,9,10-TETRAHYDRO-BENZO[A]PYREN-7-OL
- CAS Number:6272-55-5
- Molecular formula:C20H16O
- Molecular Weight:272.34
![9,10-DIHYDROBENZO[A]PYREN-7(8H)-ONE](/CAS/GIF/3331-46-2.gif)
3331-46-2
72 suppliers
$131.00/250mg
![7,8,9,10-TETRAHYDRO-BENZO[A]PYREN-7-OL](/CAS/GIF/6272-55-5.gif)
6272-55-5
42 suppliers
$43.00/250mg
Yield:6272-55-5 11.1 g
Reaction Conditions:
with sodium tetrahydridoborate in tetrahydrofuran;methanol at 20; for 1 h;
Steps:
7-Hydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (4)
The crude product 3 (12.24 g, 45.28 mmol) was dissolved in THF (150 mL, anhydrous)and CH3OH (50 mL, anhydrous) in a 500-mL round-bottom flask. NaBH4 (1.71 g,45.28 mmol) was added over 15 min, and then the reaction mixture was stirred at roomtemperature for 45 min. After checking the full conversion of 3 by TLC, the reactionwas quenched with 1N HCl, and most of the solvent was removed under reduced pressure.The residue was dissolved in CH2Cl2 (250 mL). The solution was washed withwater and then dried over MgSO4. After filtration, the solvent was evaporated underreduced pressure to afford the crude product 4 (11.10 g, 40.76 mmol, 90%). Brown-yellowsolid. 1H NMR (400 MHz, CDCl3): d 2.03-2.12 (m, 3H, CH2 and OH), 2.19-2.29(m, 2H, CH2), 3.35 (dt, J6.5, 17.06 Hz, 1H, ArCHH), 3.53 (dt, J5.8, 17.36 Hz, 1H,ArCHH), 5.22 (t, J4.7 Hz, 1H, CHOH), 7.95-8.00 (m, 3H, Ar), 8.09-8.17 (m, 3H, Ar),8.23 (d, J9.3 Hz, 1H, Ar), 8.28 (s, 1H, Ar); 13C NMR (100 MHz, CDCl3): d 18.8(CH2), 26.3 (CH2), 32.2 (CH2), 69.2 (CH), 123.0 (CH), 124.2 (C), 124.6 (C), 124.90(CH), 124.95 (CH), 125.1 (CH), 125.8 (CH), 126.7 (CH), 127.4 (CH), 127.5 (CH), 128.8(C), 129.6 (C), 130.8 (C), 130.9 (C), 131.4 (C), 136.7 (C) ppm.
References:
Kurono, Nobuhito;Iwashita, Yuji;Sugimura, Haruhiko [Organic Preparations and Procedures International,2022,vol. 54,# 5,p. 457 - 464]
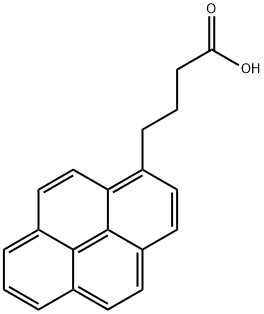
3443-45-6
212 suppliers
$40.20/1g
![7,8,9,10-TETRAHYDRO-BENZO[A]PYREN-7-OL](/CAS/GIF/6272-55-5.gif)
6272-55-5
42 suppliers
$43.00/250mg
![9,10-DIHYDROBENZO[A]PYREN-7(8H)-ONE](/CAS/GIF/3331-46-2.gif)
3331-46-2
72 suppliers
$131.00/250mg
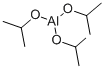
555-31-7
468 suppliers
$18.00/5g
![7,8,9,10-TETRAHYDRO-BENZO[A]PYREN-7-OL](/CAS/GIF/6272-55-5.gif)
6272-55-5
42 suppliers
$43.00/250mg

64709-55-3
28 suppliers
$151.00/1g
![7,8,9,10-TETRAHYDRO-BENZO[A]PYREN-7-OL](/CAS/GIF/6272-55-5.gif)
6272-55-5
42 suppliers
$43.00/250mg
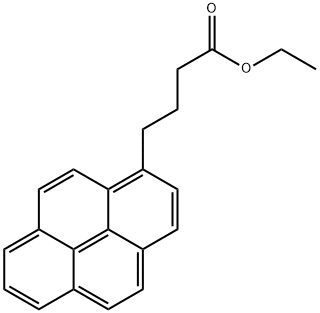
59275-39-7
2 suppliers
inquiry
![7,8,9,10-TETRAHYDRO-BENZO[A]PYREN-7-OL](/CAS/GIF/6272-55-5.gif)
6272-55-5
42 suppliers
$43.00/250mg