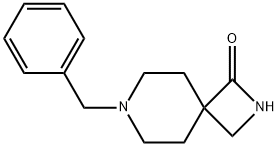
7-benzyl-2,7-diazaspiro[3.5]nonan-1-one synthesis
- Product Name:7-benzyl-2,7-diazaspiro[3.5]nonan-1-one
- CAS Number:1334536-88-7
- Molecular formula:C14H18N2O
- Molecular Weight:230.31
![2,7-Diazaspiro[3.5]nonan-1-one, 2-(phenylmethoxy)-7-(phenylmethyl)-](/CAS/20210111/GIF/1334536-87-6.gif)
1334536-87-6
2 suppliers
inquiry
![7-benzyl-2,7-diazaspiro[3.5]nonan-1-one](/CAS/GIF/1334536-88-7.gif)
1334536-88-7
26 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen in methanol at 50; under 2068.65 Torr;
Steps:
7.7D
A 2-L pressure bottle, equipped with a thermocouple and H2 inlet/outlet, was charged with Raney-nickel (7.0 g). The Raney-nickel was washed with methanol (3×, slurry and decant supernatant) and a solution of 7-benzyl-2-benzyloxy-2,7-diaza-spiro[3.5]nonan-1-one from Example 7C (28.3 g, 0.084 mol) in methanol (280 mL) was added. The reaction was placed on a Parr shaker and agitated overnight at 50° C. under an atmosphere of H2 (40 psi). The catalyst was removed by filtration and rinsed with methanol. The solution of product with benzyl alcohol was concentrated in vacuo. The benzyl alcohol was removed by azeotropic distillation, twice adding water (250 mL) and concentrating in vacuo. 2-Propanol was used to chase the water, twice adding 2-propanol (250 mL) and concentrating in vacuo to an oil. Heptane (500 mL) was added and the product was allowed to crystallize. After concentrating in vacuo to a final volume of 100 mL, the product was collected by filtration and washed with heptane (25 mL). Drying in a vacuum oven at 40° C. under a flow of N2 afforded the titled compound as a solid. 1H NMR (400 MHz, CDCl3) δ ppm 1.75-1.84 (m, 2H) 1.98-2.08 (m, 2H) 2.19-2.31 (m, 2H) 2.71-2.83 (m, 2H) 3.13 (s, 2H) 3.50 (s, 2H) 5.69 (s, 1H) 7.21-7.38 (m, 5H).
References:
US2011/230459,2011,A1 Location in patent:Page/Page column 20

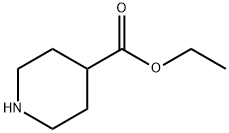
100-39-0
423 suppliers
$10.00/10g
![7-benzyl-2,7-diazaspiro[3.5]nonan-1-one](/CAS/GIF/1334536-88-7.gif)
1334536-88-7
26 suppliers
inquiry
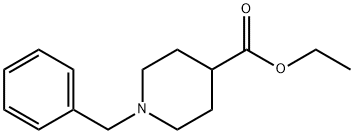
1126-09-6
484 suppliers
$15.40/25g
![7-benzyl-2,7-diazaspiro[3.5]nonan-1-one](/CAS/GIF/1334536-88-7.gif)
1334536-88-7
26 suppliers
inquiry
24228-40-8
260 suppliers
$9.59/250mg
![7-benzyl-2,7-diazaspiro[3.5]nonan-1-one](/CAS/GIF/1334536-88-7.gif)
1334536-88-7
26 suppliers
inquiry