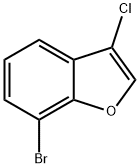
7-bromo-3-chlorobenzofuran synthesis
- Product Name:7-bromo-3-chlorobenzofuran
- CAS Number:1368229-56-4
- Molecular formula:C8H4BrClO
- Molecular Weight:231.47
![7-Bromobenzo[b]furan](/CAS/GIF/133720-60-2.gif)
133720-60-2
133 suppliers
$13.00/250mg

1368229-56-4
8 suppliers
inquiry
Yield:1368229-56-4 60%
Reaction Conditions:
Stage #1: 7-bromobenzo(b)furanwith chlorine;acetic acid at 15; for 0.5 h;
Stage #2: with potassium hydroxide in methanol at 20; for 0.333333 h;
Steps:
5.1.8 3-Chloro-7-bromobenzofuran (41)
A solution of 7-bromobenzofuran 40 (20 g, 101 mmol) in acetic acid (150 mL) was cooled to 15 °C and a gentle stream of chlorine was passed through the mixture for 0.5 h. The reaction mixture was concentrated in vacuo, diluted with water and extracted with ether (1 L). The ether layer was washed with water, dried (MgSO4), filtered and concentrated in vacuo. KOH (28 g) was dissolved in methanol (300 mL) and a solution of the crude dichlorinated compound was added dropwise into the base. The reaction mixture was stirred at room temperature for 20 min. The reaction mixture was concentrated in vacuo when a colorless solid precipitated out. The solid was filtered washed with water and dried to yield 41 (14 g, 60%) as a colorless solid. 1H NMR (400 MHz, CDCl3), δ, 7.72 (s, 1H), 7.55 (d, 1H, J = 7.6 and 1.2 Hz), 7.53 (d, 1H, J = 7.6 and 1.2 Hz), 7.21 (t, 1H, J = 7.6 Hz).
References:
Venkatraman, Srikanth;Velazquez, Francisco;Gavalas, Stephen;Wu, Wanli;Chen, Kevin X.;Nair, Anilkumar G.;Bennett, Frank;Huang, Yuhua;Pinto, Patrick;Jiang, Yueheng;Selyutin, Oleg;Vibulbhan, Bancha;Zeng, Qingbei;Lesburg, Charles;Duca, Jose;Huang, Hsueh-Cheng;Agrawal, Sony;Jiang, Chuan-Kui;Ferrari, Eric;Li, Cheng;Kozlowski, Joseph;Rosenblum, Stuart;Shih, Neng-Yang;Njoroge, F. George [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 7,p. 2007 - 2017]

95-56-7
295 suppliers
$9.00/5G

1368229-56-4
8 suppliers
inquiry
253429-15-1
11 suppliers
inquiry

1368229-56-4
8 suppliers
inquiry