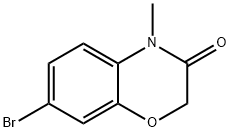
7-Bromo-4-methyl-2H-1,4-benzoxazin-3-one synthesis
- Product Name:7-Bromo-4-methyl-2H-1,4-benzoxazin-3-one
- CAS Number:1260778-66-2
- Molecular formula:C9H8BrNO2
- Molecular Weight:242.07
![7-Bromo-2H-benzo[b][1,4]oxazin-3(4H)-one](/CAS/GIF/321436-06-0.gif)
321436-06-0
125 suppliers
$24.00/250mg

74-88-4
342 suppliers
$15.00/10g

1260778-66-2
37 suppliers
$133.33/0.25g
Yield:1260778-66-2 73%
Reaction Conditions:
Stage #1: 7-bromo-3,4-dihydro-2H-1,4-benzoxazin-3-onewith potassium tert-butylate in N,N-dimethyl-formamide at 20; for 0.5 h;Inert atmosphere;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20; for 24 h;Inert atmosphere;
Steps:
7-Bromo-4-methyl-2H-benzo[b][1,4]oxazin-3(4H)-one (3a)
Potassium tert-butoxide(336 mg, 0.30 mmol) was added to a solution of 1a (342 mg, 1.50 mmol) in 2 ml dryDMF under a nitrogen atmosphere. After stirring for 0.5 h at room temperature,iodomethane (187 μl, 3.00 mmol) was added drop wise and stirring was continued for24 h. After 100 ml of water was added, the mixture was extracted three times with ethylacetate. The combined organic layers were washed with brine and dried over anhydrousMgSO4. Purification by flash chromatography (petroleum ether/ethyl acetate 4/1)afforded 3a (264 mg, 1.09 mmol, 73 %) as pale yellow solid. 1H-NMR (500 MHz,CDCl3): δ = 3.34 (s, 3H), 4.61 (s, 2H), 6.82 (d, 3J = 8.5 Hz, 1H), 7.14-7.17 (m, 2H). 13CNMR(125 MHz, CDCl3): δ = 28.1, 67.5, 115.9, 116.0, 120.1, 125.6, 128.8, 145.7, 163.9.
References:
Grombein, Cornelia M.;Hu, Qingzhong;Rau, Sabrina;Zimmer, Christina;Hartmann, Rolf W. [European Journal of Medicinal Chemistry,2015,vol. 90,p. 788 - 796] Location in patent:supporting information
367-24-8
488 suppliers
$15.00/25g

1260778-66-2
37 suppliers
$133.33/0.25g