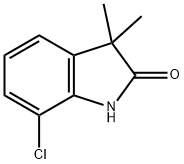
7-CHLORO-3,3-DIMETHYLINDOLIN-2-ONE synthesis
- Product Name:7-CHLORO-3,3-DIMETHYLINDOLIN-2-ONE
- CAS Number:1319743-63-9
- Molecular formula:C10H10ClNO
- Molecular Weight:195.65
25369-33-9
103 suppliers
$13.00/250mg

74-88-4
340 suppliers
$15.00/10g

1319743-63-9
7 suppliers
inquiry
Yield:1319743-63-9 61%
Reaction Conditions:
Stage #1: 7-chloro-2-oxo-1,2-dihydro-indolewith lithium dipropan-2-ylazanide in tetrahydrofuran at -40; for 0.5 h;
Stage #2: methyl iodine in tetrahydrofuran at 20; for 24 h;
Steps:
13 Intermediate Preparation Example 13: Synthesis of 7-chloro-3,3-dimethylindolin-2-one (P-13)
Dissolve 7-chloroindolin-2-one (0.34g, 2mmol) in anhydrous tetrahydrofuran (3mL), cool to -40°C, add LDA (2mol/L, 4mL, 8mmol), stir for 30min, warm to 0 , iodomethane (0.85 g, 6 mmol) was added, and the reaction was carried out at room temperature for 24 h. Saturated aqueous ammonium chloride solution (5 mL) and water (15 mL) were added to the reaction system, extracted twice with ethyl acetate (10 mL each time), the organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and reduced in pressure. After concentration, the concentrate was purified by silica gel column chromatography (petroleum ether/ethyl acetate=4:1) to obtain the title compound (0.24 g, 61%).
References:
WO2022/156351,2022,A1 Location in patent:Page/Page column 26