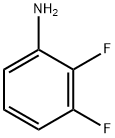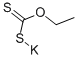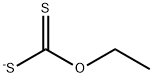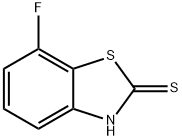
7-Fluoro-3H-benzothiazole-2-thione synthesis
- Product Name:7-Fluoro-3H-benzothiazole-2-thione
- CAS Number:154327-29-4
- Molecular formula:C7H4FNS2
- Molecular Weight:185.24
Yield:154327-29-4 100%
Reaction Conditions:
in N,N-dimethyl-formamide; for 4 h;Inert atmosphere;Heating;
Steps:
GeneralProcedure of Method B for 2-chlorobenzothiazoles Synthesis (GP3)7,8
General procedure: GP3-1: A solution of 2-halo substituted aniline (1.0 eq), potassium ethyl xanthate (1.2 eq or 2.2 eq,typically 2.2 eq) in 10 volume of anhydrous DMF was heated at 100 0Cor 120 0C for 4 hours under nitrogen. TLC monitored the progress ofreaction. After completion, the reaction mixture was cooled to room temperature,diluted with water (10 volume) and neutralized by 1 M HCl solution to pH 5. Theformed precipitate was collected by filtration, rinsed with water, firstlydried by rotavapor, and then dried by oil pump to afford 2-mercaptobenzothiazole.GP3-2: 2-mercaptobenzothiazole in 10 volume ofanhydrous DCM, was added by sulfuryl chloride (SO2Cl2, 1volume) under ice-cooled condition. The mixture was stirred at rt for 1 hour,which was monitored by TLC. After consumption of starting material, the mixturewas diluted by 30 volume of ether, following quenching carefully by addingwater. Stirring was kept for 1 hour to make sure the SO2Cl2was totally consumed and product was released. Organic layer was collected,neutralized by saturated NaHCO3, dried over Na2SO4and purified by silica gel chromatograph to give the pure product, which wasfinally characterized by LC-MS and NMR.
References:
Lv, Fengping;Li, Zhi-Fang;Hu, Wenhao;Wu, Xiaohua [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 24,p. 7661 - 7670] Location in patent:supporting information