7-hydroxy-3-(4-methoxyphenyl)-2H-chromen-2-one synthesis
- Product Name:7-hydroxy-3-(4-methoxyphenyl)-2H-chromen-2-one
- CAS Number:66267-82-1
- Molecular formula:C16H12O4
- Molecular Weight:268.26
Yield:66267-82-1 92%
Reaction Conditions:
with acetic anhydride;triethylamine at 120; for 8 h;Temperature;
Steps:
61-63 Example 61: Preparation of 3-(4-methoxyphenyl)-7-hydroxycoumarin (31)
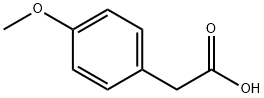
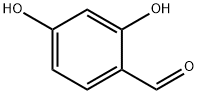
To the round bottom flask were added 4-methoxyphenylacetic acid (5g, 30.09mmol), 2,4-dihydroxybenzaldehyde (4.16g, 30.09mmol), acetic anhydride (12.29g, 120.35mmol) and triethylamine ( 6.09g, 60.18mmol), the reaction was stirred at 120°C for 8h, and the progress of the reaction was monitored by thin-layer chromatography. After the reaction is completed, according to post-treatment method C, slowly pour the reaction mixture into ice water and stir continuously to obtain a large amount of yellow solid, filter with suction, wash with water, collect the filter cake, transfer it to a round bottom flask, and then add an appropriate amount of water as a solvent. 37% concentrated hydrochloric acid (11.86g, 120.35mmol), stirred for 6h at 80°C, and monitored the progress of the reaction by thin-layer chromatography. After the reaction is complete, cool to room temperature and filter with suction to obtain a filter cake. The filter cake is washed with water until the filtrate is nearly neutral, dried, and recrystallized by ethanol-water mixed solvent to obtain 7.42 g of light yellow solid, namely 3-(4-methoxy (Phenyl)-7-hydroxycoumarin, yield: 92%.
References:
CN113121485,2021,A Location in patent:Paragraph 0193-0198
![[3-(4-methoxyphenyl)-2-oxochromen-7-yl] acetate](/CAS/20180720/GIF/66267-83-2.gif)
66267-83-2
3 suppliers
inquiry

66267-82-1
15 suppliers
inquiry

93-35-6
487 suppliers
$6.00/100mg
19501-58-7
378 suppliers
$7.00/250mg

66267-82-1
15 suppliers
inquiry
95-01-2
484 suppliers
$10.00/1g

66267-82-1
15 suppliers
inquiry