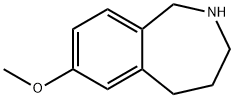
7-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[C]AZEPINE synthesis
- Product Name:7-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[C]AZEPINE
- CAS Number:159020-94-7
- Molecular formula:C11H15NO
- Molecular Weight:177.24
![7-METHOXY-2,3,4,5-TETRAHYDRO-BENZO[C]AZEPIN-1-ONE](/CAS/GIF/3648-86-0.gif)
3648-86-0
31 suppliers
$98.00/100mg
![7-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[C]AZEPINE](/CAS/20180528/GIF/159020-94-7.gif)
159020-94-7
6 suppliers
inquiry
Yield:159020-94-7 97%
Reaction Conditions:
Stage #1: 7-methoxy-2,3,4,5-tetrahydro-1H-benzo[c]azepin-1-onewith lithium aluminium tetrahydride in tetrahydrofuran at 0;Reflux;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;
Steps:
2.4
A solution of compound 2e (1.00 g, 523 mmol) in dry THF (25 mL) was added to a stirred suspension of lithium aluminum hydride (900 mg, 23.7 mmol, 4.53 eq) in dry THF (30 mL) at 0 °C. The resulting mixture was heated to reflux and allowed to stir at this temperature for 15 hours, then cooled to room temperature and quenched with aqueous 25% NaOH. A saturated solution of Na-K tartrate (200 mL) was added and the resulting solution was allowed to stir at room temperature for 2 hours. The mixture was then extracted with DCM (2 x 200 mL), the combined organic extracts were dried over MgSO4, filtered and concentrated in vacuo to provide compound 2f (903 mg, 97%) as a colorless oil
References:
WO2010/11657,2010,A1 Location in patent:Page/Page column 42
![7-methoxy-1,2,4,5-tetrahydro-benzo[c]azepin-3-one](/CAS/20180601/GIF/66191-66-0.gif)
66191-66-0
3 suppliers
inquiry
![7-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[C]AZEPINE](/CAS/20180528/GIF/159020-94-7.gif)
159020-94-7
6 suppliers
inquiry
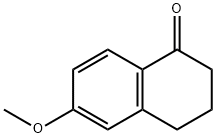
1078-19-9
363 suppliers
$6.00/5g
![7-METHOXY-2,3,4,5-TETRAHYDRO-1H-BENZO[C]AZEPINE](/CAS/20180528/GIF/159020-94-7.gif)
159020-94-7
6 suppliers
inquiry